Translate this page into:
Topical photodynamic therapy with methylaminolevulinate for the treatment of actinic keratosis and reduction of photodamage in organ transplant recipients: A case-series of 16 patients
Correspondence Address:
Ariel Hasson
Department of Dermatology, San Joaqu�n Medical Center, Facultad de Medicina, Pontificia Universidad Cat�lica de Chile, Av. Vicu�a Mackenna 4686. Macul, Santiago
Chile
| How to cite this article: Hasson A, Navarrete-Dechent C, Nicklas C, de la Cruz C. Topical photodynamic therapy with methylaminolevulinate for the treatment of actinic keratosis and reduction of photodamage in organ transplant recipients: A case-series of 16 patients. Indian J Dermatol Venereol Leprol 2012;78:448-453 |
Abstract
Background: Organ transplant recipients (OTR) are at high risk of developing cutaneous neoplasms. Topical photodynamic therapy (PDT) has been used for the treatment of actinic keratosis (AK) in OTR. Aims: The objective was to evaluate the efficacy of PDT with methylaminolevulinate (MAL) in the treatment of facial AK in OTR. As a secondary objective, we wanted to evaluate the usefulness of topical PDT in the reduction of photodamage in OTR. Methods: A prospective, single center, single arm study was made. 16 OTR were included. Topical PDT was applied for 1 or 2 cycles depending on the patient's characteristics. An evaluation of AK was made at visits pre-treatment, at 12 weeks and at 24 weeks. Photodamage was measured with multispectral image technique (SkinCare®). Results: A complete response rate of 100% was achieved for AK in all patients; it persisted without change at 12 and 24 weeks of follow-up. 62.5% of patients improved their photodamage as measured by SkinCare®, but this result was not statistically significant (P = 0.12). All patients had high level of satisfaction at the end of the therapy. Conclusions: MAL-PDT is an effective therapy for the treatment of AK in OTRs. It can reduce photodamage in this group of patients, but these results were not statistically significant.Introduction
Organ-transplant recipients (OTR) are increasing yearly worldwide. An improvement in survival and quality of life is achieved in a great number of patients with these practices. Invariably, they require the use of chronic immunosuppressive drugs to prevent organ rejection and to achieve tolerance. This carries some consequences to hosts: Adverse drug reactions, an increase in number of infections and neoplasms among others adverse events.
Cutaneous malignancies are the most common neoplasm among OTR patients. [1] Between 35% - 50% of them will develop one or more skin cancers at the 10 th year from transplantation. [2] These numbers vary among series, the type of transplant, age, the geographic area and the race of the studied population, but there is always an intrinsic increased risk. [3] Also, neoplasms have greater morbidity and mortality when compared with general population with an increase in aggressiveness and capacity to metastasize in these patients. [2]
Squamous cell carcinoma (SCC) is the most frequent skin cancer in OTR population; with an inverse SCC to basal cell carcinoma (BCC) ratio of 4:1 compared to the 1:4 in general population. [4] SCC occurs 65 to 250 times its normal incidence in this group of patients. Actinic keratosis (AK) incidence is also increased in OTR patients with an occurrence of 35% after 5 years of immunosuppression. [5],[6]
The formation of AK could be explained by the formation of thymine dimers and p53 mutations in keratinocytes. [7] Other genes mutated frequently in AK are the ras genes, c-myc, and p16 among others. [8] Human papilloma virus (HPV) and its role in the development of AK in OTR has also been documented. [9]
Aggressive and early treatment is essential to prevent the progression of AK to invasive SCC. Many therapies have been validated for the treatment of AK including cryotherapy, topical 5-fluorouracil (5-FU), curettage; imiquimod and electrosurgery among others. [5] Many of these treatments can accomplish a high cure-rate, but their use is limited by the great number of lesions in OTR and because of esthetic secondary effects like scars.
In the last years, photodynamic therapy (PDT) has emerged as an alternative for the treatment of AK in immunocompetent patients. [10],[11],[12],[13] The tumoricidal mechanism of action of PDT is through free oxygen radical species, generated by a photosensitizer activated by visible light. [14] Topical photosensitizers like 5-aminolevulinic acid (ALA) and 5-methylaminolevulinate (MAL) are metabolized by cells to protoporphyrin IX a molecule that is capable of causing oxidative damage by itself when activated by visible light. [10]
The methyl-esterified formulation of ALA, MAL has greater lipophilicity and could penetrate deeper in the skin. [12] Both agents are relatively selective and concentrate in target tissue probably because of an altered superficial permeability, caused by the alteration in the cutaneous barrier and because of the altered metabolism of porphyrins by tumor cells. [12]
The objective of the present study was to evaluate the efficacy of photodynamic therapy in OTR with facial AK. As a secondary objective, we wanted to evaluate if there was an improvement in the photodamage level at the end of the treatment in these patients.
Methods
47 OTR patients were on preventive follow-up in our department and were recruited for the study. 31 patients were excluded because they did not have facial AK. 16 patients finally entered the study.
It was a prospective clinical study. Patients were followed-up between April and October 2007 in Hospital Clínico de la Pontificia Universidad Católica de Chile, Chile.
We included only AK of the face and scalp; if patients had AK in other locations, they were not included in the study and were treated with conventional treatments. Diagnosis was made clinically when they were evident, and when diagnosis was not clear, samples were sent for histology.
Exclusion criteria were porphyria and known allergy to MAL or its components. AK lesions, capable of being treated, were followed up with clinical appreciation before treatment, at 12 weeks and 24 weeks post-treatment.
The follow-up was done by the same investigators of the study without blinding.
Complete response (CR) was considered when no residual lesions were observed clinically after treatment. A partial response (PR) was considered when < 50% of initial lesions were present after follow-up, and failure to response (FR) was considered when there were >50% of initial lesions were present.
Cleaning of the skin was done using physiologic solution (0.9% normal saline). In hypertrophic lesions, superficial curettage was done before starting treatment. We did not use topical anesthetics. Then, MAL 160 mg/g (Metvix® , Photocure ASA, Oslo, Norway) was applied. A 1 mm thickness with 5 mm of margin of MAL was applied and incubated with occlusive dressing (Tegaderm® 3M, USA) for 3 hours. Then, the patch was removed, and MAL was cleaned with physiologic solution. The area was then irradiated at a distance of 8 centimeters with a LED coherent light lamp (Aktilite® , model CL16 and CL128) with visible red light, wavelength of 635 nm at 37 J/cm 2 . The number of sessions was decided individually for every patient. If at 12-weeks, AK lesions persisted or were still >50% of basal, a new MAL-PDT session was done.
The level of photodamage was evaluated with Skin Care® (Clarity Pro Facial Stage, Moritex, USA), which captures a multispectral image and automatically evaluates photodamage level in a desired area without contrasting with healthy skin. It was evaluated only on the face. Then it was classified as low: 0 - 13 points, moderate: 14 - 27 points, and severe: 28 - 50 points. Photodamage was measured before treatment and at 12- or 24- weeks of follow-up.
Adverse events were evaluated objectively and subjectively, including pain and tolerance to illumination. They were registered after the PDT session and after a week of treatment. We also decided to evaluate patient satisfaction subjectively at 12 weeks of follow-up with the simple question: Considering results and adverse events you suffered, are you satisfied with the treatment? Patients should answer yes or no.
Statistical analysis was made using MINITAB 15 (Minitab INC, 2007) with the t-test for paired samples. We used a confidence level of 95%.
Results
Mean age in the study was 49 years (20 - 72 years). 12 patients were men (Male:Female: 3:1). Of all patients, 14 patients were renal transplanted, 1 patient was heart transplanted, and 1 patient was hepatic transplanted. The mean time since they had organ transplant was 12.5 years (range: 1 - 25 years).
Phototype was evaluated according to Fitzpatrick phototyping scale. 2 patients were type II phototype, 7 patients were type III, and 7 patients were type IV phototype.
A total of 16 patients were treated with MAL-PDT. All the lesions were on the face or on the scalp. After patients′ follow-up at 12 and 24 weeks, a CR was obtained in all patients (16/16) . There was no PR or FR [Table - 1]. 6 patients (37.5%) needed a second session to obtain CR because at 12-week follow-up, there were still AK lesions.
All patients described good tolerance to the discomfort felt while being illuminated and all the patients enlisted finished the determined number of sessions.
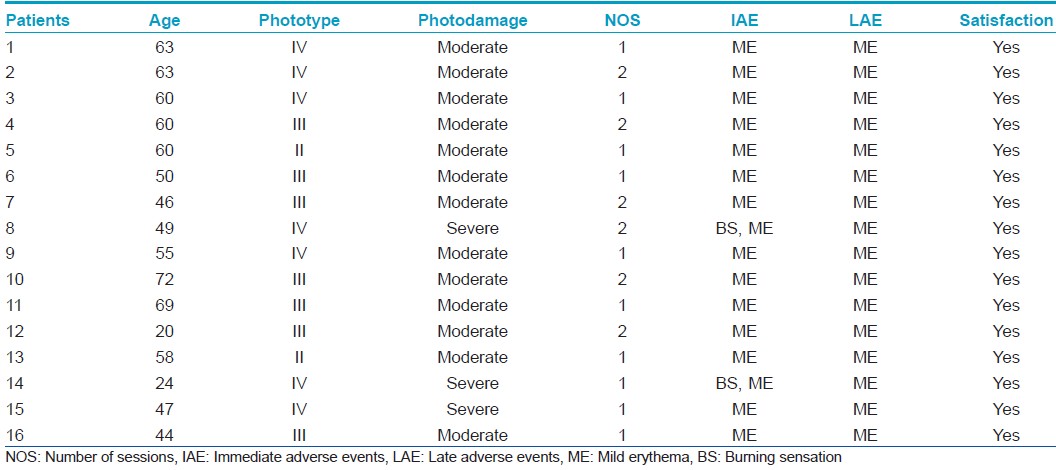
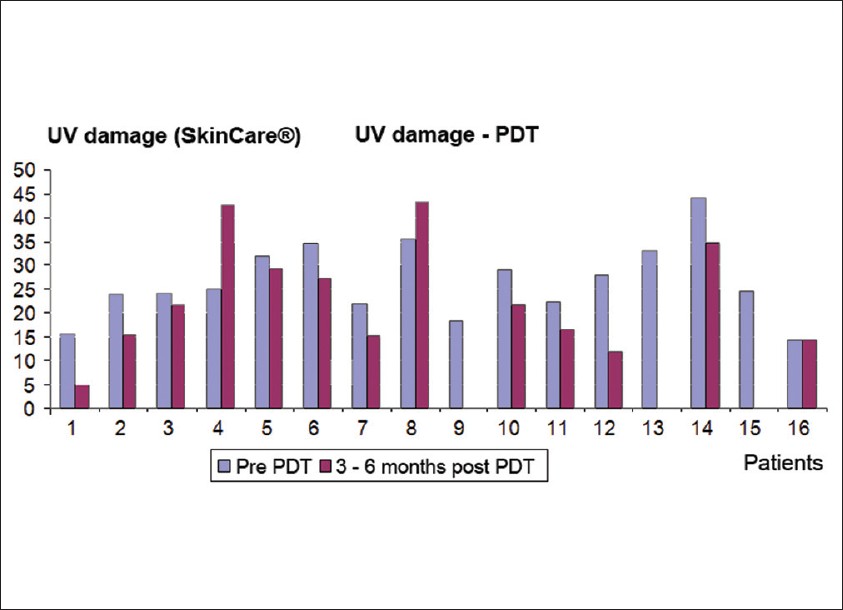
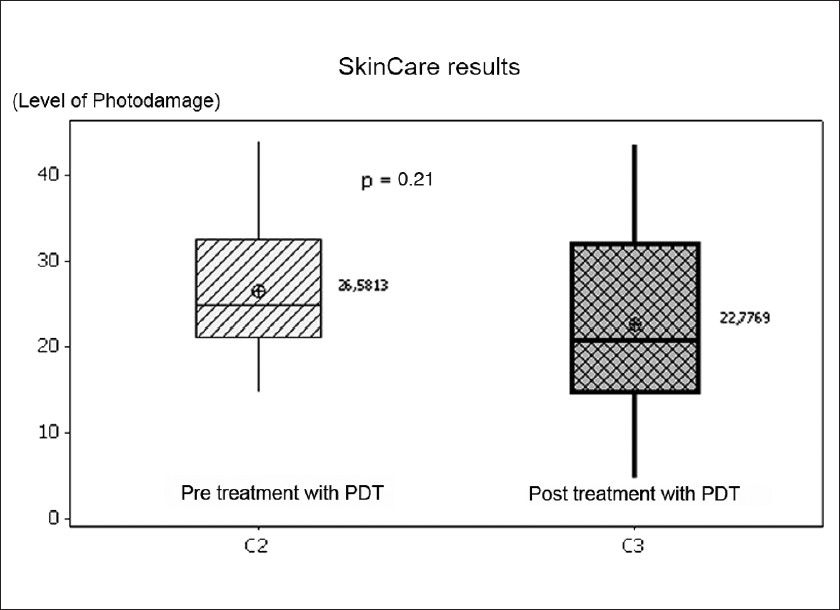
Photodamage was measured at a basal level and at 12 and 24 weeks [Figure - 1]. 13 patients had moderate photodamage and 3 patients had severe photodamage at the beginning of the study. Pre-treatment mean was 26 ± 8.1 points. Post-treatment (combined at 24 weeks), the mean was 22 ± 11.9 points. These differences were not statistically significant [P-value = 0.21, [Figure - 2], [Figure - 3], [Figure - 4] and [Figure - 5]. Differences in photodamage were also evaluated separately according to skin phototype: Patients with phototype IV (7 patients) and patients with phototype III or less (9 patients) also had no statistically significant improvement (P = 0.21 and P = 0.349, respectively). 3 patients were not measured because they were lost at follow-up, but they were included in the analysis.
 |
| Figure 1: Photodamage - Skin Care at basal level and at 3 and 6 months after photodynamic therapy. Photodamage level: low: 0 - 13 points, moderate: 14 - 27 points, severe: 28 - 50 points |
 |
| Figure 2: Photodamage mean and standard deviation pre-treatment and post-treatment with MAL-PDT |
 |
| Figure 3: SkinCare multispectral photodamage evaluation of patient number 1 and 12. (a) Shows patient 1 before MAL-PDT. (b) Shows patient 1 after MAL PDT. (c) Shows patient 12 before MAL-PDT. (d) Shows patient 12 after MAL PDT. Photodamage level: low: 0 - 13 points, moderate: 14 - 27 points, severe: 28 - 50 points |
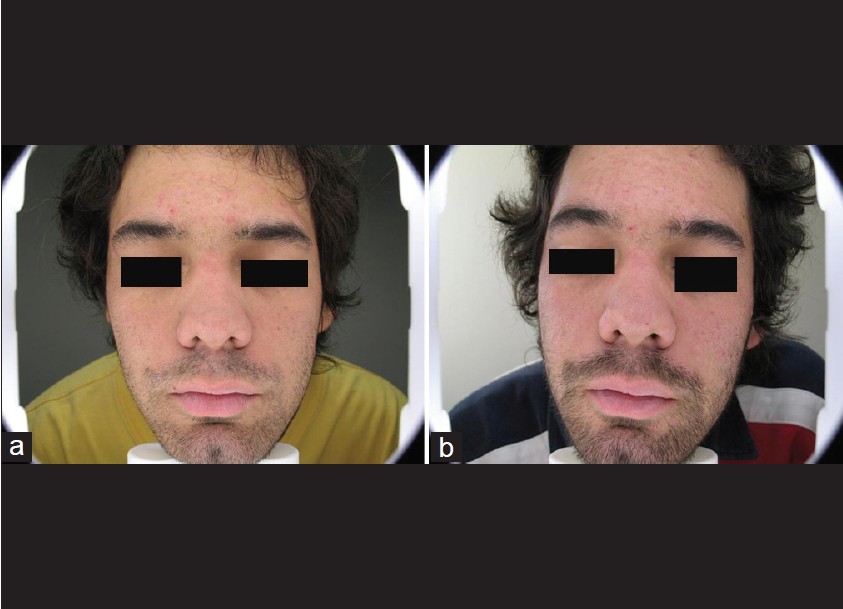 |
| Figure 4: Patient number 12. Note frontal lesions. (a) Before topical MAL-PDT treatment. (b) After treatment with topical MAL-PDT treatment |
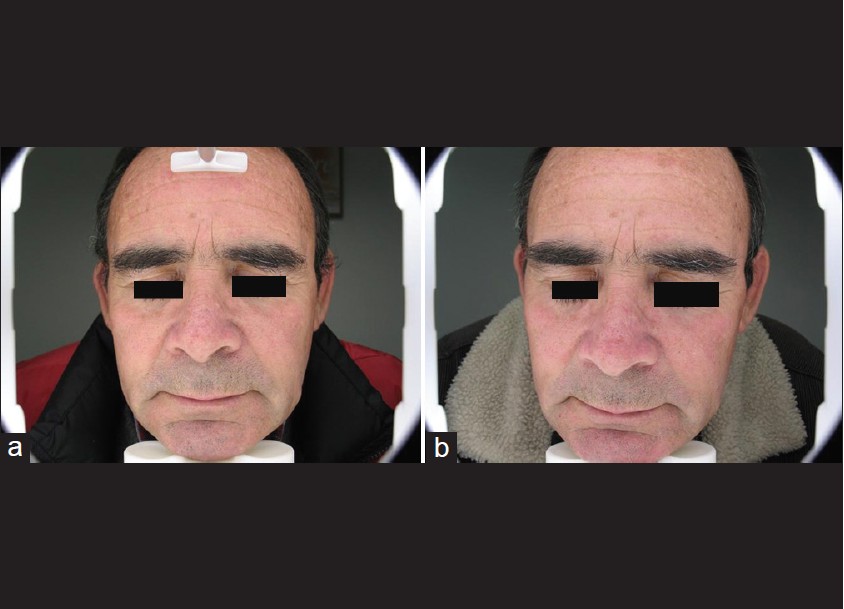 |
| Figure 5: Patient number 10. Note frontal lesions. (a) Before topical MAL-PDT treatment. (b) After treatment with topical MAL-PDT |
As an immediate adverse reaction, all the patients reported mild erythema, which resolved spontaneously. 2 patients reported moderate burning sensation, which resolves after an administration of 500 mg of acetaminophen. Late reaction was defined if it occurred after the first week of treatment; all the patients had mild erythema, thus not needing treatment.
Pain was measured with a visual analog scale (range 0 - 10), 1 patient (6%) reported intensity of pain as 1/10, and 11 patients (68%) reported it as 2/10; 3 patients (18%) reported it as 3/10 and only 1 patient (6%) reported an intensity of pain as 4/10.
Of the 6 patients that received 2 PDT sessions, 5 of them (83%) evaluated their pain as 1/10, and only 1 patient (16%) reported an intensity of pain as 2/10 in visual analog scale. At the end of the study, all patients had high level of satisfaction (100% patients responded "yes").
Discussion
AK and non-melanoma skin cancer (NMSC) are one of the most frequent complications of long term immunosuppression in OTR. Its frequency has been estimated 50 - 200 times more often than the general population. [15] Most relevant risk factors are UV radiation and the degree and duration of immunosuppressive treatment. [16] More evidence has been added in the last years that AK may even be a very superficial SCC or that it can be considered an in situ SCC. [17] Additionally, in OTR patients, the progression of AK to SCC could be even faster than in immunocompetent individuals. [18] For these reasons, AK should be actively treated in this group of patients.
In this study, we tested the utility of MAL-PDT in the treatment of AK in OTR. Our results show a complete response in all the treated patients. These results are consistent with those published by others who showed a 71% - 90% response rate with MAL-PDT for AK in renal transplant recipients with a similar PDT protocol. [5],[18] Our results are also concordant with the published guidelines, [10],[11],[12] showing a similar response rate to PDT in OTR group and in immunocompetent group of 86% versus 94%, respectively. Nevertheless, at long-term follow-up, the response rates could be lower for the OTR group. [12] Our response-rate evaluations were only made with physical examination and were not made with skin biopsy; we are aware that we may be overestimating MAL-PDT efficacy in OTR.
MAL-PDT has also been compared to conventional therapies, like topical 5-FU with a complete response of 89% in the MAL-PDT group and no complete response in any patient in the 5-FU group at 1 month follow-up. At 3 months of follow-up, complete responses were conserved in the PDT group. [19]
Our study also described the change in cutaneous photodamage, measured by a multispectral image method at a basal, 12 and 24 weeks after topical MAL PDT. An improvement was observed in 62.5% of patients, but these results were not statistically significant (P = 0.12). Differences were also non-significant when evaluated separately according to skin phototype. Also, 12.5% of patients worsened their photodamage level in the follow-up. We conducted this photodamage evaluation searching for a statistically significant improvement in photodamage, but this could not be demonstrated. We think this may be due to the low number of patients in the study, causing great standard deviations. However, there is a tendency for an improvement in the photodamage level at 12 and 24 weeks.
To our knowledge, this is the first report of photodamage evaluation, with an objective measuring method as a multispectral image using MAL-PDT in transplanted patients. Others have evaluated the appearance of new AK in the PDT group versus the control group with a tendency of reduction in the number of AK in the treated group but without significant differences. [20] However, Wulf et al. showed that 38% of the patients treated with PDT developed new premalignant lesions at 12 months, but 38% of patients with new lesions occurring at 9.6 months versus 6.8 months in the control group. [21] Wulf et al. used a similar treatment protocol (red light, MAL and curettage of hyperkeratotic lesions) to ours. It is known that photodamaged skin expresses Ki-67, p53 [22] and cyclin D1. [23] Bagazgoitia et al. [24] conducted a study to evaluate the improvement in early oncogenic markers using PDT in AK patients (not OTR). They found a reduction in the basal keratinocytic dysplasia and elastosis; they found a reduction in Ki-67 expression, p53 and a non-significant reduction in cyclin D1. This could be an indirect indicator of the utility of PDT as a preventive measure for photodamaged skin in OTR patients.
Regarding the adverse events reported, we did not have any severe adverse event or esthetic consequences in our series. Nor did we have any patient withdrawn from the study because of this reason. All patients showed mild erythema that persisted in all of them at 1 week follow-up. All of them also referred pain, but most of the patients characterized it as a mild pain, and it seems that it diminished at the second session. The adverse events reported by our group are similar to those reported elsewhere. [10],[12] It seems that PDT is a safe therapy in this group of patients. All patients were satisfied in this study.
Our study has the weakness of lacking a control group, therefore, we cannot exclude that this results could be obtained with placebo, [20] but this seems difficult because there are many studies that validate the use of PDT-MAL as a therapy for AK in OTR. [5],[10],[12],[18],[19],[21],[24]
The number of transplants and the number of recipients with better immunosuppressive therapies and better management of chronic pathologies will increase every day. It also seems that the use of new immunosuppressant drugs may lower the incidence of AK and non melanoma skin cancer (NMSC), new therapeutic alternatives like sirolimus could open new opportunities to OTR. Preliminary studies showed a reduction in the incidence of NMSC with the use of these drugs. [15] Upcoming studies will clarify this possibility. A large number of available treatments are at hand once lesions have already appeared. Topical PDT appears to be effective for treating AK in transplanted patients and emerges as an option. PDT could also reduce photodamage in OTR patients as supported by this study and could become a preventive opportunity in this group of patients.
Conclusion
Topical MAL-PDT is an effective and well-tolerated treatment alternative for AK in OTR, with only mild adverse events. It also seems effective as a preventive measure by reducing the level of photodamage in these patients. More studies are required to establish efficacy, security and a protocol scheme in OTR patients.
For the time being, we recommend a collaborative work with all the health team to offer these patients the best available options. In this myriad of options, topical PDT appears as an effective and tolerable treatment alternative.
| 1. |
Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin Cancer in Organ Transplant Recipients -- Where Do We Stand Today? Am J Transplant 2008;8:2192-8.
[Google Scholar]
|
| 2. |
Donovan JC, Rosen CF, Shaw JC. Evaluation of sun-protective practices of organ transplant recipients. Am J Transplant 2004;4:1852-8.
[Google Scholar]
|
| 3. |
Sandoval M, Ortiz M, Díaz C, Majerson D, Molgó M. Cutaneous manifestations in renal transplant recipients of Santiago, Chile. Transplant Proc 2009;41:3752-4.
[Google Scholar]
|
| 4. |
Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348:1681-91.
[Google Scholar]
|
| 5. |
Dragieva G, Prinz BM, Hafner J, Dummer R, Burg G, Binswanger U, et al. A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolevulinate in the treatment of actinic keratoses in transplant recipients. Br J Dermatol 2004;151:196- 200.
[Google Scholar]
|
| 6. |
Perera GK, Child FJ, Heaton N, O'Grady J, Higgins EM. Skin lesions in adult liver transplant recipients: A study of 100 consecutive patients. Br J Dermatol 2006;154:868-72.
[Google Scholar]
|
| 7. |
Stoff B, Salisbury C, Parker D, O'Reilly Zwald F. Dermatopathology of skin cancer in solid organ transplant recipients. Transplant Rev (Orlando) 2010;24:172-89.
[Google Scholar]
|
| 8. |
Padilla RS, Sebastian S, Jiang Z, Nindl I, Larson R. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: A spectrum of disease progression. Arch Dermatol 2010;146:288-93.
[Google Scholar]
|
| 9. |
Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: Advances in therapy and management Part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 2011;65:253-61.
[Google Scholar]
|
| 10. |
Morton CA, Brown SB, Collins S, Ibbotson S, Jenkinson H, Kurwa H, et al. Guidelines for topical photodynamic therapy: Report of a workshop of the British Photodermatology Group. Br J Dermatol 2002;146:552-67.
[Google Scholar]
|
| 11. |
Christensen E, Warloe T, Kroon S, Funk J, Helsing P, Soler AM, et al. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J Eur Acad Dermatol Venereol 2010;24:505-12.
[Google Scholar]
|
| 12. |
Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: Update. Br J Dermatol 2008;159:1245- 66.
[Google Scholar]
|
| 13. |
Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: Advances in therapy and management Part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 2011;65:263-79.
[Google Scholar]
|
| 14. |
Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: Basic principles and present clinical experience. J Photochem Photobiol B 1990;6:143-8.
[Google Scholar]
|
| 15. |
Salgo R, Gossmann J, Schöfer H, Kachel HG, Kuck J, Geiger H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: Reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant 2010;10:1385-93.
[Google Scholar]
|
| 16. |
Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002;47:1-17.
[Google Scholar]
|
| 17. |
Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol 2006;155:9-22.
[Google Scholar]
|
| 18. |
Piaserico S, Belloni Fortina A, Rigotti P, Rossi B, Baldan N, Alaibac M, et al. Topical photodynamic therapy of actinic keratosis in renal transplant recipients. Transplant Proc 2007;39:1847-50.
[Google Scholar]
|
| 19. |
Perrett CM, McGregor JM, Warwick J, Karran P, Leigh IM, Proby CM, et al. Treatment of post-transplant premalignant skin disease: A randomized intrapatient comparative study of 5-fluorouracil cream and topical photodynamic therapy. Br J Dermatol 2007;156:320-8.
[Google Scholar]
|
| 20. |
de Graaf YG, Kennedy C, Wolterbeek R, Collen AF, Willemze R, Bouwes Bavinck JN. Photodynamic therapy does not prevent cutaneous squamous-cell carcinoma in organ-transplant recipients: Results of a randomized-controlled trial. J Invest Dermatol 2006;126:569-74.
[Google Scholar]
|
| 21. |
Wulf HC, Pavel S, Stender I, Bakker-Wensveen CA. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol 2006;86:25- 8.
[Google Scholar]
|
| 22. |
Carpenter PM, Linden KG, McLaren CE, Li KT, Arain S, Barr RJ, et al. Nuclear morphometry and molecular biomarkers of actinic keratosis, sun-damaged, and nonexposed skin.Cancer Epidemiol Biomarkers Prev 2004;13:1996-2002.
[Google Scholar]
|
| 23. |
Narbutt J, Norval M, Slowik-Rylska M, Jochymski C, Kozlowski W, Sysa-Jedrzejowska A, et al. Suberythemal ultraviolet B radiation alters the expression of cell cycle-related proteins in the epidermis of human subjects without leading to photoprotection. Br J Dermatol 2009;161:890-6.
[Google Scholar]
|
| 24. |
Bagazgoitia L, Cuevas Santos J, Juarranz A, Jaén P. Photodynamic therapy reduces the histological features of actinic damage and the expression of early oncogenic markers. Br J Dermatol 2011;165:144-51.
[Google Scholar]
|
Fulltext Views
3,150
PDF downloads
2,951





