Translate this page into:
Tumor necrosis factor-α antagonists: Side effects and their management
Correspondence Address:
Sunil Dogra
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Dogra S, Khullar G. Tumor necrosis factor-α antagonists: Side effects and their management. Indian J Dermatol Venereol Leprol 2013;79:35-46 |
Abstract
As elevated levels of tumor necrosis factor-alpha (TNF-α) are associated with disease severity in psoriasis and psoriatic arthritis, TNF-α antagonists are being used to treat moderate to severe disease in patients who have contraindications, fail to respond or develop side effects to conventional systemic therapies. It is of utmost importance to be well versed with the possible adverse effects and contraindications of TNF-α antagonists so that they can be used effectively and safely. Many of their adverse effects have been well studied in patients of rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) and may not be completely applicable in psoriasis. This is because patients with RA and IBD are on multiple immunosuppressants while those with psoriasis are mostly receiving single systemic therapy and often have comorbidities that distinguish them from those with RA or IBD. Also, some of the side effects are still controversial and debated. Long-term prospective randomized controlled studies are needed to better understand the associated risk in patients of psoriasis. Baseline screening and periodic monitoring during treatment can reduce and help in early identification and appropriate management of the adverse outcomes. This article reviews the side effects known to be associated with TNF-α antagonists, their pathomechanisms and management guidelines. Some of the common side effects include infusion and injection site reactions, infections particularly reactivation of tuberculosis, autoantibody formation and drug induced lupus erythematosus, liver function abnormalities, hematological, and solid organ malignancies.Introduction
Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine that plays a prominent role in the pathogenesis of psoriasis and psoriatic arthritis (PsA), rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and many other dermatologic and rheumatologic conditions. It is bound to the cell surface in its precursor transmembrane form (tmTNF-α) and is released from cells after cleavage as soluble TNF-α (sTNF-α). Both forms are biologically active, and bind to either of the two receptors, TNF receptor 1 (TNFR1, p55) and TNF receptor 2 (TNFR2, p75). [1] Currently there are five biologic therapies that target TNF: infliximab, a chimeric human-murine monoclonal antibody; adalimumab and golimumab, fully human anti-TNF-α monoclonal antibodies (IgG1); etanercept, a fusion protein composed of a dimer of the extracellular portions of human TNFR2 (p75) fused to the Fc domain of human IgG1; and certolizumab, a pegylated, humanized Fab fragment of an anti-TNF-α monoclonal antibody. [2]
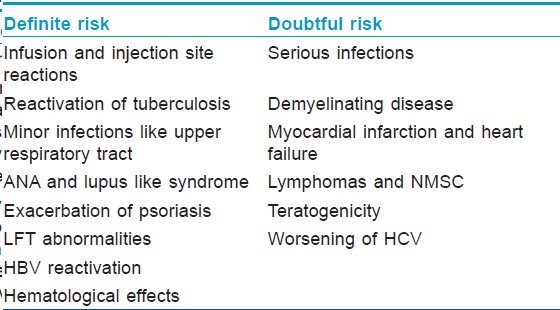
These drugs have been in use in rheumatology for quite some time and have proven their efficacy for treatment of psoriasis too. As these drugs are increasingly being employed, many of their adverse effects are being recognized, some novel and some paradoxical. The data regarding safety profile of TNF-α antagonists in psoriasis and PsA is sparse. In order to conclusively confirm the existing controversial side effects as well as those recently documented, it is necessary that all adverse effects of any degree of severity be reported. This will require that psoriatic patients are registered and followed up for years along with the control population so as to obtain long-term data regarding associated risk with their prolonged use. The establishment of British Association of Dermatologists Biologic Interventions Registry (BADBIR) that registers all psoriatic patients receiving biologic therapy in United Kingdom and follows them up for 5 years is an important step in reporting and maintaining this vital information. [Table - 1] categorizes the associated side effects into either definite or doubtful risk. The common side effects and their management have been summarized in this review.
Infusion Reactions
Infusion reactions are characteristically seen with infliximab as it is the only TNF-α antagonist administered intravenously. Acute infusion reactions occur within 24 h of infusion, generally within 2 h. The manifestations include fever, chills, sweating, headache, pruritus, nausea, flushing, dizziness, dyspnoea, chest pain, hypotension or hypertension, and anaphylaxis. Delayed reactions occur between 24 h and 14 days (majority in 5-7 days) after an infusion, with symptoms including arthralgia, myalgia, rash, and fatigue. [3] Infusion reactions can also be categorized as mild, moderate, or severe. While most reactions are mild to moderate, severe ones such as anaphylactoid and serum sickness-like reactions occur in about 1% of patients.
Pathomechanisms
Anaphylactoid reactions result from direct degranulation of mast cells by the drug and are not mediated by IgE. Delayed reactions are not characterized by laboratory findings of immune complexes and are therefore named as serum sickness like based on typical symptoms. As infliximab is a chimeric molecule with mouse derived variable sequences, formation of antibodies to infliximab (ATI) is higher than with adalimumab and etanercept. ATI increase the risk of infusion reactions and decrease the efficacy of treatment. [3] The incidence of reactions in antibody positive cases was 40% in contrast to 4.7% in ATI negative patients in a randomized controlled trial (RCT) in Crohn′s disease. [4] The risk of reactions with ATI is concentration dependent and has been shown to increase at 8 μg/ml and beyond. [5]
Management
Management of infusion reactions includes both prevention and treatment.
Prevention
Premedication
Premedication with paracetamol, antihistamines, and corticosteroids may prevent the development of infusion reactions. In a double-blind RCT, hydrocortisone group experienced fewer reactions compared to placebo (24% vs. 15%) but the difference was not statistically significant. [4]
Infusion schedule
Induction therapy with three infusions at 0, 2, and 6 weeks is associated with significantly less drug specific antibodies compared to a single infusion. [6] It has also been shown in a RCT that maintenance treatment every 8 weeks lessens the number of infusion reactions compared to on demand treatment in psoriasis. [7]
Immunosuppressants
Concurrent treatment with methotrexate decreases antibody formation and hence the incidence of infusion reactions compared to infliximab monotherapy (40% vs. 16%) in Crohn′s disease. [8]
Treatment
Mild to moderate reactions
The infusion rate should be reduced to 10 ml/h or stopped. Paracetamol and antihistamines should be administered and the vital signs monitored every 10 minutes. If the infusion is stopped, it is restarted at 10 ml/h after 20 minutes and increased by 20 ml/h every 15 minutes up to a maximum of 120 ml/h.
Severe reactions
The infusion should be stopped and the vital signs monitored every 2 minutes. If the systolic blood pressure falls below 95 mmHg or if wheezing occurs, adrenaline 0.5 mg IM should be given. Intravenous corticosteroids, antihistamines, and normal saline are also needed for anaphylactoid reactions.
The decision to continue infliximab in a patient with a history of infusion reaction depends on the severity of reaction, the efficacy of treatment, and the treatment substitutes available. For mild or moderate reactions, premedication, immunosuppressants and adjustment of schedule and rate of infusions may decrease the probability of a future reaction. The next infusion should be started at 10 ml/h and slowly increased to a maximum of 125 ml/h. [9],[10] For delayed reactions, increasing the dose or reducing the dosing interval decreases the formation of soluble complexes. [3] Desensitization is considered in severe reactions in the absence of alternative therapy.
Injection site Reactions
Injection site reactions (ISRs), including erythema, itching, pain, swelling, and hemorrhage, are the most common adverse effects of etanercept and adalimumab therapy. In a review of RCTs in patients with moderate to severe psoriasis, ISRs were observed in 18.3% with 50 mg twice weekly etanercept, 17% with 25 mg twice weekly, 16.3% with 50 mg once weekly, and 11% with 25 mg once weekly. [11] The frequency in PsA was 36%. [12] Most reactions occurred in the first 3 months of treatment. As they were mild to moderate in severity, they resolved without stopping the drug. [11]
Pathomechanisms
The two mechanisms underlying development of ISRs are irritative and immune-mediated. [11] Irritative reactions appear after the first injection and resolve without discontinuation of treatment. They are related to a high concentration of the drug, improper injection technique, and vehicle components. Immune mediated reactions, including type I and type III hypersensitivity, require the formation of anti-etanercept antibodies and hence occur after the second injection onwards following sensitization. The frequency of these antibodies varied from 0.6% to 18.3% in RCTs in patients with psoriasis, RA, and ankylosing spondylitis. [11] Formation of a new antigen due to unrelated human amino acid sequences at the fusion site is responsible for site specific antibody production. These antibodies do not affect treatment efficacy as they are non-neutralizing. Although adalimumab is a fully humanized monoclonal antibody, new immunogenic sites may be formed by the adalimumab-TNF-α complex, thus resulting in fewer reactions compared to etanercept. The majority of reactions are mild to moderate, do not recur, and subside without drug discontinuation.
Management
Skin testing with a prick test should be done initially and if negative, an intradermal test should be performed starting with 1:100 dilution and read at 20-30 minutes for type I reactions and at 6 and 24 h for type III reactions. If the immediate reading is positive, etanercept should be stopped to avoid systemic anaphylactic reactions with subsequent injections. If alternative therapies are not available, desensitization should be considered. Subcutaneous desensitization with etanercept was performed successfully in a patient of ankylosing spondylitis with positive immediate intradermal test. Oral aspirin 325 mg, montelukast 10 mg, diphenhydramine 25 mg, and famotidine 40 mg were given 1 h prior to desensitization along with daily cetrizine during desensitization. On days 1 and 2, increasing doses of etanercept starting at 0.25 mg in 1:100 dilution were administered up to 4.5 mg in 1:1 dilution with a total of 12.25 mg. On day 3, 0.5 mg in dilution of 1:10 was started, doubled up to 8.5 ml and total of 24.75 ml was given. On day 4, 25 mg in 1:1 dilution was administered. [13] Delayed reactions subside with subsequent injections due to immune tolerance and etanercept can be continued in such cases without loss of efficacy. [11]
Infections
Tuberculosis
Latent tuberculosis infection (LTBI) is an asymptomatic infection by Mycobacterium tuberculosis. A Spanish study found its incidence to be 29% (42/144) based on positive tuberculin skin test (TST) and chest radiographs in psoriatic patients screened for anti-TNF-α therapy, and this was comparable with the general population. [14] As TNF-α plays an important role in the host defense against tuberculosis, progression of LTBI to active tuberculosis is a major concern with anti-TNF-α therapies, particularly in endemic countries. The risk is greater with infliximab (1.5/1000 patient years) and adalimumab (0.9/1000 patient years) than with etanercept (0.5/1000 patient years). [15] Unusual presentations like extrapulmonary and disseminated forms are seen in around 50% of cases. [16],[17] The latent period between initiation of therapy and development of clinical symptoms is around 3 months, [16],[17] 4-6 months, [18] and 11.5 months [19] for infliximab, adalimumab, and etanercept, respectively.
Management
All patients to be started on anti-TNF-α therapy should be screened for the presence of LTBI. The TST, based on a delayed type hypersensitivity response to purified protein derivative (PPD), was the standard test to detect LTBI until recently. Induration measuring 5 mm or more at 48-72 h is taken as the cut-off value for a positive result according to Centers for Disease Control and Prevention (CDC). [20] However, in Bacillus Calmette Guerin (BCG) vaccinated individuals, induration of 15 mm or more should be considered positive according to British Thoracic Society recommendations. [21] The National Psoriasis Foundation (United States) in 2008 recommended exposure history, TST and chest radiography for screening of LTBI prior to treatment with TNF-α antagonists and annual screening for those on long-term therapy. [22] It has been reported that exaggerated and false positive responses to the TST may be seen in psoriatic individuals due to a proinflammatory dermis resulting in Koebnerization and that TST positivity correlates with the disease severity score, thus arousing concern regarding its utility in these patients. [23] Other drawbacks of TST include low specificity in BCG-vaccinated individuals (if vaccinated <15 years before TST) and those infected with nontuberculous mycobacteria, decreased sensitivity in immunocompromised individuals, inability to distinguish LTBI from active TB, second visit for reading of result, and subjective interpreter variability. Interferon-gamma release assays (IGRAs) like the QuantiFERON-TB test, the QuantiFERON-TB Gold test (QFT-G), the QuantiFERON-TB Gold In-Tube test, and the T-SPOT TB test have now been approved by the US Food and Drug Administration (FDA) to aid in the diagnosis of latent and active tubercular infection. These in vitro blood tests measure the production of IFN-γ by T cells exposed to antigens like early secretory antigen target-6, and culture filtrate protein-10, which are specific for Mycobacterium tuberculosis. Other advantages of IGRAs over TST include higher sensitivity particularly in immunocompromised population, [24] single patient visit, results within 24 h, and no boosting of results with subsequent tests. The disadvantages include high cost, limited availability, errors in blood collection or transport, blood processing required within 8-16 h of collection and difficulty in predicting who with positive test will develop active infection. Guidelines by CDC in 2010 recommend that IGRAs may be used instead of TST to screen for LTBI. However, both tests may be required in the following situations: (a) high suspicion of infection, but the initial test with IGRA is negative; (b) low risk of infection but the test is positive; or (c) if initial IGRA is indeterminate, as can occur in immunocompromised patients or those at extremes of age (< 5 years or >80 years). IGRA is preferred in BCG-vaccinated individuals and those unlikely to return for TST reading while TST should be used for children < 5 years of age. [25] With respect to live vaccines, IGRA should be performed either on the same day or 4-6 weeks after vaccination. Following TST, IGRA should be performed within 3 days to avoid boosting of results. [26] Negative TST and positive QTF-G test have been reported in patients on long-term anti-TNF-α treatment, thus recommending QFT-G as the primary test in these patients. [27]
Treatment with daily isoniazid 300 mg for 9 months is recommended for LTBI. Anti-TNF-α therapy should preferably be started after completion of chemoprophylaxis. However, if the patient is compliant and well tolerating the prophylaxis, therapy can be started after 1-2 months depending on the severity of disease. [22] A complete course of prophylaxis is effective in preventing active tuberculosis in about 70% of patients. [28] It is therefore important to monitor patients receiving anti-TNF-α therapy for signs of active tuberculosis and if present, therapy should be stopped and an antitubercular regimen initiated accordingly.
Other Infections
Among other potential infections with TNF-α inhibitors, upper respiratory tract infections, bronchitis, and urinary tract infections are the most common. Serious infections like pneumonia, diverticulitis, pyelonephritis, and postsurgical infections are uncommon and more likely in patients with underlying predisposing medical conditions. [29] However, the data regarding the risk of serious infections is controversial. While some studies suggest no increased risk, [30],[31] a meta-analysis of RCTs in RA concluded that there was a 2-fold increase in the incidence of serious infections with anti-TNF-α therapy as compared to placebo. [32] Opportunistic infections such as histoplasmosis, legionellosis, salmonellosis, listeriosis, coccidioidomycosis, cryptococcosis, aspergillosis, candidiasis, and pneumocystosis have been reported more commonly with infliximab or adalimumab than with etanercept in RA. However, many of these patients were also receiving other immunosuppressive agents. [33],[34],[35]
Neurological
The issue of TNF-α antagonists leading to development or worsening of neurological disease is unsettled. Patients with multiple sclerosis have high concentrations of TNF-α in the serum and cerebrospinal fluid (CSF). A systematic review evaluating demyelinating diseases in patients with rheumatic diseases treated with TNF-α antagonists concluded that the rate of demyelinating diseases in patients treated with TNF-α antagonists does not differ from the expected rate in the population. [36]
However, TNF-α antagonists have been reported to cause neurological events suggestive of demyelination. A detailed review of neurological symptoms reported to the FDA in 2001 identified 17 cases due to etanercept and 2 due to infliximab therapy in inflammatory arthritides. The symptoms were temporally related to initiation of therapy and resolved on discontinuation with recurrence in one case following rechallenge. The most common symptom was paraesthesia, followed by visual disturbance due to optic neuritis. Other symptoms included gait disturbances, confusion, apraxia, facial palsy, and Guillain-Barre syndrome. It has been proposed that there may be a chance coexistence of autoimmune diseases like multiple sclerosis and inflammatory arthritis and genetic susceptibility to develop these may get unmasked with anti-TNF-α therapies. [37]
Management
According to American Academy of Dermatology guidelines, TNF-α antagonist therapy should be avoided in patients with a personal history of or a first-degree relative with a demyelinating disorder. [38] If neurological symptoms suggestive of demyelination develop during therapy, treatment should be withdrawn. The following steps should be further taken:
- Neurologic examination, including fundus for papilledema and optic neuritis.
- Magnetic resonance imaging (MRI) of the brain with and without gadolinium.
- Lumbar puncture for CSF studies, including oligoclonal bands and IgG levels.
- If the above investigations are inconclusive or the patient has rapid clinical and/or radiographic progression, a brain biopsy may be helpful to delineate the differential diagnosis.
- Therapies for multiple sclerosis such as glucocorticoids, IFN-γ, or intravenous immunoglobulin (IVIG) should be considered.
Although autoantibody formation following anti-TNF-α therapy is common, anti-TNF-α induced lupus is rare. A review of open-label and randomized placebo-controlled trials of anti-TNF-α biologics in RA showed that antinuclear antibody (ANA) positivity increased from 29% pretreatment to 53% posttreatment. [39] Among those treated with infliximab, 14% developed anti-dsDNA antibodies, the majority being IgM antibodies. One patient developed a reversible lupus-like clinical syndrome (0.6%) associated with IgM, IgA, and IgG anti-dsDNA antibodies. These autoantibodies are usually of low titer and generally not accompanied with clinical symptoms. They are associated with a higher incidence of infusion reactions. Though all three can induce antibody formation, infliximab is more likely to do so than etanercept and adalimumab. Assessment of these antibodies using multiplexed fluorescent microsphere immunoassay (MFMI), indirect immunofluorescence (IIF), and enzyme-linked immunosorbent assay (ELISA) methods found them to be anti-SS-B, antiribonucleoprotein (anti-RNP), anti-Sm, anti-Jo-1, and antihistone, with anti-SS-B being the most frequent. [40] In a survey from France, 22 patients were found to have lupus induced by anti-TNF-α therapy for rheumatic diseases, of which 12 patients had full blown lupus, satisfying 4 out of 11 ARA criteria. Anti-TNF-α induced lupus was seen in 0.19% and 0.18% of infliximab and etanercept treated patients, respectively. [41] Anti-TNF-α lupus has been found to differ from classical drug induced lupus, with more frequent cutaneous, renal, and CNS involvement. [42],[43] Proposed mechanisms for the formation of autoantibodies include shift from Th1 to Th2 cytokine production, increased T cell apoptosis, resulting in presentation of nucleosomal antigens, and lowering of C-reactive protein that interferes with removal of nuclear debris. [44],[45]
Management
The production of antibodies and thereby lupus can be prevented by concomitant administration of disease modifying antirheumatic drugs (DMARDs). However, no beneficial effect was seen in patients receiving both methotrexate and infliximab for RA. [39] TNF-α antagonists can be continued if there is only antibody formation and no clinical symptoms. Discontinuation of treatment results in resolution of symptoms. The Spanish Study Group of Biological Agents in Autoimmune Diseases (BIOGEAS) divided patients into two groups, mild and severe. Mild disease with cutaneous and articular symptoms was controlled with withdrawal of anti-TNF-α therapy, while severe disease with lung, renal, and CNS features was in addition treated with immunosuppressants. Symptoms disappeared in 94% of cases after withdrawal of anti-TNF-α therapy alone. Corticosteroids and other immunosuppressants were required in 40% and 12% patients, respectively. [43] A retrospective observational study in psoriatic patients who received anti-TNF-α therapy found that 83% of patients who had failed to respond to all three treatments (infliximab, etanercept, and adalimumab) developed ANA and anti-dsDNA antibodies in contrast to 17% and 2%, respectively, in those who had not failed any treatment. The antibodies appeared before treatment failure and were not related to duration of therapy. These antibodies may be prospectively measured after initiation of treatment to predict therapeutic failure to anti-TNF-α agents. [46]
Worsening of Psoriasis
New development of psoriasis or paradoxical worsening of existing psoriasis has been reported with anti-TNF-α therapies after days to years. Inhibition of TNF-α stimulates plasmacytoid dendritic cells (PDCs) to produce increased levels of IFN-α. Early lesions and normal skin of psoriatic patients express PDCs with IFN-α expression in lesional blood vessels. This results in expression of chemokine receptors on Th1 lymphocytes and their subsequent homing to the skin. [47]
In a review of 104 patients with psoriatic skin lesions due to anti-TNF-α treatment, 52% developed pustular psoriasis, 49%, plaque psoriasis, and 16%, guttate psoriasis. About 50% of patients were treated for RA, 22% for seronegative spondyloarthropathy, and 16% for IBD. The majority of cases (53%) were due to infliximab, followed by etanercept (29%) and adalimumab (18%). Eight patients had a history of psoriasiform lesions. Nine patients with preexisting psoriasis experienced worsening of their disease or developed new morphology of lesions. Anti-TNF-α therapy was continued in 79% patients with either the same drug or another one of the same class. [48]
Management
In patients with severe and progressive disease, anti-TNF-α therapy should be stopped and treatment for psoriasis initiated accordingly. Anti-TNF-α therapy can be continued for those with mild disease [i.e., involving < 5% body surface area (BSA)], and psoriasis treated with topical agents. If the disease covers >5% BSA or if there is palmoplantar pustulosis, switching to an alternative TNF-α antagonist is considered along with topical occlusive therapies for psoriasis. For uncontrolled disease, ultraviolet phototherapy, methotrexate, cyclosporine, or acitretin may be administered. [48]
Some of the less common cutaneous adverse effects including leucocytoclastic vasculitis, eczema, and lichenoid reactions have been reported in RA patients. [49] Serious reactions like erythema multiforme, -Stevens-Johnson syndrome, and rarely toxic epidermal necrolysis have also been described with the use of TNF-α antagonists in RA. However, 71% of these patients were concomitantly receiving other medications associated with these reactions. [50] Development of toxic epidermal necrolysis is paradoxical as TNF-α antagonists have been successfully used in its treatment.
Hepatic Abnormalities
Liver function test (LFT) abnormalities, mostly asymptomatic and transient, have been reported with anti-TNF-α agents. Infliximab-induced elevation in liver enzymes without any symptoms occurred in 8% patients treated for psoriasis. [51] Rarely, severe liver involvement, in the form of autoimmune hepatitis and acute liver failure, has been associated with infliximab. [52] Administration of anti-TNF-α agents is not a contraindication in patients with chronic hepatitis of any etiology. However, extreme caution is required in cirrhotic patients as there is increased risk of liver decompensation due to drug-induced hepatic injury.
Management
Baseline LFTs should be done before starting anti-TNF-α treatment. If the LFTs are normal and the patient does not have an underlying risk of developing liver disease, the LFTs need not be monitored during the course of treatment in the absence of symptoms or signs of hepatotoxicity. If the baseline LFTs are normal but the patient has risk factors for liver disease, LFTs should be repeated every 12 weeks. [53] If the liver enzymes become elevated during therapy but less than three times the upper limit, treatment is continued. Treatment is continued with caution and LFTs monitored periodically if values are between 3 and 5 times of normal and stopped if >5 times the normal limit.
TNF-α and HCV Infection
Treatment of chronic inflammatory conditions with anti-TNF-α agents in the setting of hepatitis C virus (HCV) infection is a challenge as the viral load may increase with worsening of hepatitis. High levels of TNF-α are associated with markedly elevated transaminases and lesser response to anti-HCV therapy. [54] Treatment with etanercept, the most commonly used agent in a systematic review of 153 patients treated with anti-TNF-α agents for psoriasis, RA, and Crohn′s disease in the setting of HCV infection, resulted in one confirmed and five suspected cases of deterioration of hepatitis among 110 patients. Etanercept treatment also resulted in stabilization of liver enzymes and viral load in 74 patients and an improvement in HCV associated chronic liver disease in combination with IFN-γ and ribavirin therapy in 29 patients. [55] Though the risk of hepatotoxicity varies between different TNF-α antagonists, etanercept seems to be well tolerated in HCV positive patients.
Management
All patients should be screened for HCV infection before starting treatment with TNF-α antagonists and a hepatologist consulted for appropriateness of therapy in positive cases. Eligible patients should be investigated with a pretreatment liver biopsy, monthly aminotransferase levels, and periodic quantitative HCV RNA load. A liver biopsy is indicated if aminotransferase levels are persistently elevated and HCV viral load >1 log. [56],[57] Prospective trials evaluating the safety of long-term treatment with anti-TNF-α therapies in chronic HCV infection are needed.
TNF-α and HBV Infection
Elevated levels of TNF-α occur in the serum and hepatocytes of patients with chronic hepatitis B infection. It plays a role in inhibiting viral replication and clearance of viral infected hepatocytes. Hence, anti-TNF-α therapy can result in reactivation of liver disease. The spectrum of clinical reactivation ranges from an asymptomatic self-limiting hepatitis to severe and potentially fatal involvement.
Management
The risk of hepatitis B virus (HBV) reactivation stresses the need for screening for HBV markers (HBsAg, -anti-HBs, anti-HBc) in all patients in need of anti-TNF-α therapy. In active HBV carriers, antiviral therapy with the nucleoside/nucleotide analogues entecavir or tenofovir is recommended before starting anti-TNF-α regimens and needs to be continued lifelong or until clearance of HbsAg. In inactive HBV carriers, universal prophylaxis with lamivudine is indicated prior to anti-TNF-α regimens and needs to be continued for at least 6-12 months after completion of immunosuppressive therapy. Viremia should be monitored after treatment completion for early detection of reactivation. In HBsAg-negative, anti-HBc-positive carriers, TNF-α inhibitors are safe since the risk of HBV reactivation is very low. Close monitoring of HBsAg every 3 months and antiviral therapy started upon evidence of HBV reactivation may be advisable in these patients. [58]
Heart diseases
The association of TNF-α antagonists with cardiac complications is controversial. RCTs like anti-TNF-α Therapy Against Chronic Heart Failure (ATTACH), Randomized Etanercept Wordwide Evaluation (RENEWAL), Randomized Etanercept North American Strategy to Study Antagonism of Cytokines (RENAISSANCE), and Research into Etanercept Cytokine Antagonism In Ventricular Dysfunction (RECOVER) that evaluated anti-TNF-α agents in patients with clinically stable New York Heart Association (NYHA) class III-IV heart failure concluded that heart disease worsened in these patients. [59] Hence, TNF-α antagonists are absolutely contraindicated in patients with NYHA class III-IV heart failure. A screening echocardiogram should be done in well-compensated (NYHA class I and II) cardiac failure and if ejection fraction is < 50% of normal, TNF antagonists should not be given.
Low serum levels of TNF-α, if present prior to cardiac ischemia, play an important role in the reduction of infarct size and in cardiac remodeling and repair. Anti-TNF-α agents, by preventing these cardioprotective functions of TNF-α, could worsen severe heart failure. [60] Contrary to this, a systematic review evaluating the incidence of cardiovascular events associated with TNF-α antagonists in patients with RA found no statistically significant increased risk of myocardialinfarction, heart failure, and stroke. [61] Most patients enrolled had no history of preexisting heart failure and development of new onset heart failure was not demonstrated. Systemic proinflammatory mediators like TNF-α contribute to increased incidence of atherosclerosis in RA. [62] By reducing inflammation, anti-TNF-α agents reduce the risk of cardiovascular disease and are not associated with a risk of heart failure in patients with RA.
Malignancy
Most of the studies that have evaluated the incidence of malignancies with TNF-α antagonists have been conducted in patients of RA and IBD. In a meta-analysis of nine trials in patients with RA treated with infliximab or adalimumab, the pooled odds ratio for malignancy was noted to be 3.3 (95% CI 1.2-9.1). The rates were greater for higher than for lower doses. [63] However, this may not be true for psoriasis as RA patients are on multiple immunosuppressive drugs that synergistically increase the rate of malignancies with TNF-α antagonists. A meta-analysis of RCTs examining the risk of malignancies in psoriatic patients found no statistically significant increased risk of malignancy. Most malignancies found during the placebo-controlled portions of the trials were nonmelanoma skin cancer (70.6%). [64] However, long-term studies are needed to assess the risk of cancer with anti-TNF-α therapy in psoriasis. It has been shown that the baseline risk of lymphoma (Hodgkin′s and cutaneous T-cell lymphoma) irrespective of treatment is high in patients with psoriasis as for RA. [65] The risk of cutaneous malignancies is further increased in patients with a history of treatment with psoralen and ultraviolet-A (PUVA )and cyclosporine. Hepatosplenic T cell lymphoma has been reported in Crohn′s disease treated with infliximab and adalimumab along with azathioprine or mercaptopurine. [66]
In a study assessing the risk of tumors with TNF-α blockers in RA patients, 757 patients who received etanercept or infliximab were compared with 800 patients with conventional antirheumatic treatment. The relative risk of total tumors, excluding lymphomas, in the anti-TNF-α and comparison groups were 0.79 and 1.39, respectively, while that of lymphomas was 11.5 and 1.3, respectively. This suggests that the risk of lymphomas was significantly increased with anti-TNF-α therapy independent of the risk with RA per se. [67]
Management
Despite the controversial results regarding the risk of malignancies, including nonmelanoma skin cancers (NMSC) and lymphoma with TNF-α antagonists, all patients and particularly those with history of multiple immunosuppressive therapy should be screened for any potential malignancy before and during anti-TNF-α therapy. Skin and lymph node examination should be carried out at baseline, 6 monthly for first year and yearly thereafter. [68]
Hematological
Thrombocytopenia
Thrombocytopenia is a rare side effect of TNF-α antagonists and significant thrombocytopenia (i.e., platelet count <50,000/mm 3 ) is even rarer. Several pathogenic mechanisms have been proposed. These include formation of antiplatelet antibodies similar to autoimmune thrombocytopenia or systemic lupus erythematosus (SLE), direct drug-induced destruction of platelets, decreased bone marrow production or increased aggregation. [69] According to a review, 19 cases of thrombocytopenia have been reported, 9 of whom were suffering from psoriasis or PsA. There was no history of concurrent administration of other immunosuppressive agent in 84% of patients. Infliximab was the culprit drug in 53%, etanercept in 42%, and adalimumab in 10% of cases. The duration of exposure varied from 1 to 67 weeks. Platelet count <50,000 was present in 53% of patients. [70]
Neutropenia
Neutropenia is defined as an absolute neutrophil count < 1500/mm 3 . The risk of infections rises significantly with counts <1000/mm 3 . The mechanisms for neutropenia include suppression of hematopoiesis by inhibiting bone marrow differentiation, antibodies against neutrophils, and neutrophil precursor suppression by large T lymphocytes. A recent review reported 111 cases of neutropenia due to anti-TNF-α therapy, RA being the underlying diagnosis in most cases (83%); psoriasis and PsA were present in 1 and 7 patients, respectively. Neutropenia occurred in the absence of another myelosuppressive drug in 96% of patients. Etanercept was most commonly implicated (72.8%), followed by infliximab (18.5%) and adalimumab (9%). Suggested risk factors were low baseline neutrophil count (<4 × 10 9 /L) and history of neutropenia with other DMARDs. Severe neutropenia (<500/mm 3 ) was seen in seven cases. Serious infections like osteomyelitis and pneumonia occurred in only 6% of patients. Initial treatment was continued in 81% of patients, while the remaining were shifted to another anti-TNF-α agent. [70]
Management
Therapy is discontinued if the neutrophil count is <500 mm 3 . The British Society of Rheumatology has recommended regular blood count (total and differential leukocyte count) monitoring at baseline and during the course of treatment in patients on anti-TNF-α monotherapy or in combination with other DMARDs.
Thromboembolism
The association between TNF-α antagonists and thromboembolic events is not clear. TNF-α activates the coagulation pathway by stimulating the secretion of IL-6. The formation of anti-phospholipid and anti-cardiolipin antibodies has also been associated with thrombosis due to anti-TNF-α therapy. [71],[72] TNF-α is also known to play a role in atherosclerotic plaque formation. However, the confounding role of factors like immobilization, smoking, and concomitant drugs cannot be completely excluded in these patients. Venous thrombosis is relatively more common (77.5%) than arterial thrombosis (22.5%). The majority of cases had IBD and RA (12% each), while psoriasis and PsA accounted for 5.5% of patients. [70]
Development of a thrombotic event should be investigated using tests like antinuclear and anticardiolipin autoantibodies, antithrombin III, homocysteine, protein C and S, prothrombin and factor V Leiden mutations, and lipid profile.
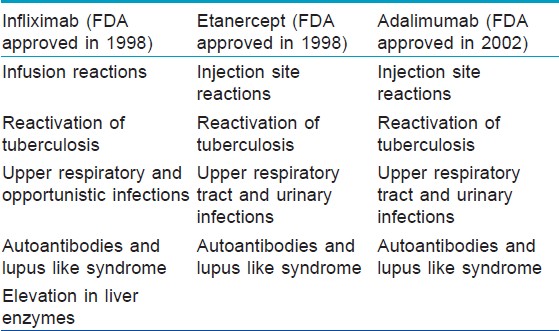
Relatively uncommon hematological adverse effects reported include pancytopenia, aplastic anemia, [73] hypereosinophilia, [74] adalimumab-induced granulomatous disease of the bone marrow, [75] infliximab-induced autoimmune hemolytic anemia, [76] and macrophage activation syndrome. [77] [Table - 2] enlists the common side effects with each of the three TNF-α antagonists.
Teratogenicity and Breast Feeding
TNF-α antagonists are classified as pregnancy category B drugs and animal studies have not shown evidence of toxicity to fetus. There are limited data regarding the outcome of pregnancy in humans. Cases of miscarriage in RA [78] and preterm birth in Crohn′s disease [79] have been reported. In contrast, there is a series of patients who have delivered normal babies while on treatment with anti-TNF-α therapies. [80] A recent review of the FDA database evaluating the risk of congenital abnormalities with the use of anti-TNF-α agents found 61 congenital anomalies in 41 newborn babies, of which 22 patients were on etanercept and 19 on infliximab therapy. Heart defects were the most common and 59% babies had one or more features of vertebral abnormalities, anal atresia, cardiac defect, tracheoesophageal, renal, and limb abnormalities (VACTERL) syndrome. [81] While infliximab has not been detected in breast milk of lactating mothers, [82] etanercept has been reported to be excreted in breast milk. [83] According to the current evidence, TNF-α antagonists should not be administered during pregnancy and lactation. Female patients of reproductive age group must use effective methods of contraception while on anti-TNF-α therapy. If pregnancy occurs during therapy, close monitoring of the fetus is required. [84]
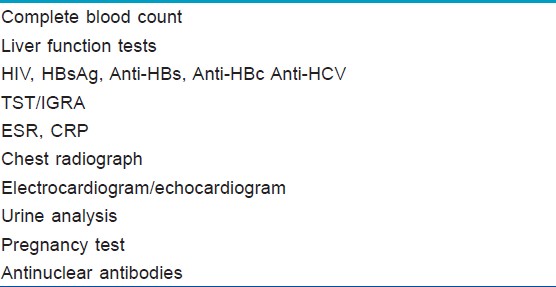
[Table - 3] summarizes the essential laboratory investigations before treatment with TNF-α antagonists.
Conclusion
The relative recent introduction of TNF-α antagonists in the treatment of psoriasis warrants complete awareness of the incidence and management of common and rare side effects associated with their use. The safety profile of anti-TNF-α therapy is conflicting with regard to major organ systems, serious infections, and malignancies. It is important to weigh the risk of the therapy with the likelihood of disease improvement in a given patient. As more clinical experience and long-term data on their safety is available, it will be possible to use them more judiciously in future.
| 1. |
Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA et al. British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987-1019.
[Google Scholar]
|
| 2. |
Sivamani RK, Goodarzi H, Garcia MS, Raychaudhuri SP, Wehrli LN, Ono Y et al. Biologic therapies in the treatment of psoriasis: A comprehensive evidence-based basic science and clinical review and a practical guide to tuberculosis monitoring. Clin Rev Allergy Immunol 2013;44:121-40.
[Google Scholar]
|
| 3. |
Cheifetz A, Mayer L. Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med 2005;72:250-6.
[Google Scholar]
|
| 4. |
Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: A randomized controlled trial. Gastroenterology 2003;124:917-24.
[Google Scholar]
|
| 5. |
Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003;348:601-8.
et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003;348:601-8. '>[Google Scholar]
|
| 6. |
Candon S, Mosca A, Ruemmele F, Goulet O, Chatenoud L, Cézard JP. Clinical and biological consequences of immunization to infliximab in pediatric Crohn's disease. Clin Immunol 2006;118:11-9.
[Google Scholar]
|
| 7. |
Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2007;56:31.e1-15.
[Google Scholar]
|
| 8. |
Vermeire S, Noman M, van Assche G, Baert F, D'Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut 2007;56:1226-31.
[Google Scholar]
|
| 9. |
Lequerre T, Vittecoq O, Klemmer N, Goëb V, Pouplin S, Menard JF, et al. Management of infusion reactions to infliximab in patients with rheumatoid arthritis or spondyloarthritis: Experience from an immunotherapy unit of rheumatology. J Rheumatol 2006;33:1307-14.
[Google Scholar]
|
| 10. |
Han PD, Cohen RD. Managing immunogenic responses to infliximab: Treatment implications for patients with Crohn's disease. Drugs 2004;64:1767-77.
[Google Scholar]
|
| 11. |
Batycka-Baran A, Flaig M, Molin S, Ruzicka T, Prinz JC. Etanercept-induced injection site reactions: potential pathomechanisms and clinical assessment. Expert Opin Drug Saf 2012;11:911-21.
[Google Scholar]
|
| 12. |
Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264-72.
[Google Scholar]
|
| 13. |
Bavbek S, Aydin O, Ataman S, Cahill K, Castells M. Injection-site reaction to etanercept: Role of skin test in the diagnosis of such reaction and successful desensitization. Allergy 2011;66:1256-7.
[Google Scholar]
|
| 14. |
Sanchez-Moya AI, Dauden E. Incidence of tuberculosis infection in psoriatic patients on antieTNF therapy: Report of a case series with 144 patients. J Eur Acad Dermatol Venereol 2011;25:730-3.
[Google Scholar]
|
| 15. |
Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:2368-76.
[Google Scholar]
|
| 16. |
Lim WS, Powell RJ, Johnston ID. Tuberculosis and treatment with infliximab. N Engl J Med 2002;346:623-6.
[Google Scholar]
|
| 17. |
Keane J, Gershon SK, Wise R. Tuberculosis associated with infliximab, a tumor necrosis factor a-neutralizing agent. N Engl J Med 2001;345:1098-104.
[Google Scholar]
|
| 18. |
Hernandez Cruz B, Cetner AS, Jordan JE, Puangsuvan SN, Robinson JK. Tuberculosis in the age of biologic therapy. J Am Acad Dermatol 2008;59:363-80.
[Google Scholar]
|
| 19. |
Mohan N, Cote TR, Block JA, Manadan AM, Siegel JN, Braun MM. Tuberculosis following the use of etanercept, a tumour necrosis factor inhibitor. Clin Infect Dis 2004;39:295-9.
[Google Scholar]
|
| 20. |
Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol 2006;2:602-10.
[Google Scholar]
|
| 21. |
British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005;6:800-5.
[Google Scholar]
|
| 22. |
Doherty SD, Van Voorhees A, Lebwohl MG, Korman NJ, Young M, Hsu S. National Psoriasis Foundation consensus statement on screening for latent tuberculosis infection in patients with psoriasis treated with systemic and biologic agents. J Am Acad Dermatol 2008;59:209-17.
[Google Scholar]
|
| 23. |
Tsiouri G, Gaitanis G, Kiorpelidou D, Dionysiou A, Efthymiou A, Daskalopoulos G, et al. Tuberculin skin test overestimates tuberculosis hypersensitivity in adult patients with psoriasis. Dermatology 2009;219:119-25.
[Google Scholar]
|
| 24. |
Kim EY, Lim JE, Jung JY, Son JY, Lee KJ, Yoon YW, et al. Performance of the tuberculin skin test and interferon-gamma release assay in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis 2009;9:207.
[Google Scholar]
|
| 25. |
Centers for Disease Control and Prevention. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Morb Mortal Wkly Rep 2010;59:1-28.
[Google Scholar]
|
| 26. |
Sanchez-Moya AI, Dauden E. Diagnosing latent tuberculosis infection in patients with psoriasis under antitumour necrosis factor-a treatment: Every new solution breeds new doubts. Br J Dermatol 2011;164:204-30.
[Google Scholar]
|
| 27. |
Haddican MM, Koo JY. Is tuberculin skin testing reliable during anti-tumor necrosis factor-alfa therapy? A case report and review of the literature. J Am Acad Dermatol 2011;65:195-7.
[Google Scholar]
|
| 28. |
Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 2005;52:1766-72.
[Google Scholar]
|
| 29. |
Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol 2009;23 Suppl 2:S1-70.
[Google Scholar]
|
| 30. |
Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving antitumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:2368-76.
[Google Scholar]
|
| 31. |
Schneeweiss S, Setoguchi S, Weinblatt ME, Katz JN, Avorn J, Sax PE, et al. Antitumor necrosis factor Alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum 2007;56:1754-64.
[Google Scholar]
|
| 32. |
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-85.
[Google Scholar]
|
| 33. |
Lee JH, Slifman NR, Gershon SK, Edwards ET, Schwieterman WD, Siegel JN, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002;46:2565-70.
[Google Scholar]
|
| 34. |
Slifman NR, Gershon SK, Lee JH, Edwards ET, Braun MM. Listeria monocytogenes infection as a complication of treatment with tumor necrosis factor alpha-neutralizing agents. Arthritis Rheum 2003;48:319-24.
[Google Scholar]
|
| 35. |
Wallis RS, Broder M, Wong J, Lee A, Hoq L. Reactivation of latent granulomatous infections by infliximab. Clin Infect Dis 2005;41 Suppl:S194-8.
[Google Scholar]
|
| 36. |
Fernández-Espartero MC, Pérez-Zafrilla B, Naranjo A, Esteban C, Ortiz AM, Gómez-Reino JJ et al. Demyelinating disease in patients treated with TNF antagonists in rheumatology: Data from BIOBADASER, a pharmacovigilance database, and a systematic review. Semin Arthritis Rheum 2011;40:330-7.
[Google Scholar]
|
| 37. |
Mohan N, Edwards ET, Cupps TR, Oliverio PJ, Sandberg G, Crayton H, et al. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum 2001;44:2862-9.
[Google Scholar]
|
| 38. |
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50.
[Google Scholar]
|
| 39. |
Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: Findings in open-label and randomized placebo-controlled trials. Arthritis Rheum 2000;43:2383-90.
[Google Scholar]
|
| 40. |
Caramaschi P, Ruzzenente O, Pieropan S, Volpe A, Carletto A, Bambara LM, et al. Determination of ANA specificity using multiplexed fluorescent microsphere immunoassay in patients with ANA positivity at high titres after infliximab treatment: preliminary results. Rheumatol Int 2007;27:649-54.41.
[Google Scholar]
|
| 41. |
De Bandt M, Sibilia J, Le Loët X, Prouzeau S, Fautrel B, Marcelli C, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: A French national survey. Arthritis Res Ther 2005;7:545-51.
[Google Scholar]
|
| 42. |
Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alpha agents. Semin Arthritis Rheum 2008;37:381-7.
[Google Scholar]
|
| 43. |
Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies. Analysis of 233 cases. Medicine 2007;86:242-51.
[Google Scholar]
|
| 44. |
Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy. Immunol Res 1999;20:147-61.
[Google Scholar]
|
| 45. |
Lorenz HM, Herrmann M, Winkler T, Gaipl U, Kalden JR. Role of apoptosis in autoimmunity. Apoptosis 2000;5:443-9.
[Google Scholar]
|
| 46. |
Pink AE, Fonia A, Allen MH, Smith CH, Barker JN. Antinuclear antibodies associate with loss of response to antitumour necrosis factor-a therapy in psoriasis: A retrospective, observational study. Br J Dermatol 2010;162:780-5.
[Google Scholar]
|
| 47. |
De Gannes GC, Ghoreishi M, Pope J, Russell A, Bell D, Adams S, et al. Psoriasis and pustular dermatitis triggered by TNF-inhibitors in patients with rheumatologic conditions. Arch Dermatol 2007;143:223-31.
[Google Scholar]
|
| 48. |
Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: A literature review and potential mechanisms of action, Arthritis Rheum 2008;59:996-1001.
[Google Scholar]
|
| 49. |
Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL. Dermatological conditions during TNFalpha- blocking therapy in patients with rheumatoid arthritis: A prospective study. Arthritis Res Ther 2005;7:R666.
[Google Scholar]
|
| 50. |
United States Food and Drug Administration: Tumor necrosis factor alpha (TNF-a) antagonists [infliximab (marketed as REMICADE), etanercept (marketed as ENBREL), and adalimumab (marketed as HUMIRA)]: Serious skin reactions. FDA Drug Saf Newsl 2008;1:18-22.
[Google Scholar]
|
| 51. |
Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: A phase III, multicentre, double-blind trial. Lancet 2005;366:1367-74.
[Google Scholar]
|
| 52. |
Infliximab (Remicade) [package insert]. Centocor Inc, Malvern, PA, 2006.
[Google Scholar]
|
| 53. |
Huang W, Cordoro KM, Taylor SL, Feldman SR. To test or not to test? An evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis. J Am Acad Dermatol 2008;58:970-7.
[Google Scholar]
|
| 54. |
Calabrese LH, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: Hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis 2004;63:18-24.
[Google Scholar]
|
| 55. |
Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of anti-tumour necrosis factor agents in patients with chronic hepatitis C infection: A systematic review. Rheumatology (Oxford) 2011;50:1700-11.
[Google Scholar]
|
| 56. |
Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009;49:1335-74.
[Google Scholar]
|
| 57. |
Pontisso P, Bellati G, Brunetto M, Chemello L, Colloredo G, Di Stefano R, et al. Hepatitis C virus RNA profiles in chronically infected individuals: Do they relate to disease activity? Hepatology 1999;29:585-9.
[Google Scholar]
|
| 58. |
Vigano M, Degasperi E, Aghemo A, Lampertico P, Colombo M. Anti-TNF drugs in patients with hepatitis B or C virus infection: Safety and clinical management. Expert Opin Biol Ther 2012;12:193-207.
[Google Scholar]
|
| 59. |
Coletta AP, Clark AL, Banarjee P, Cleland JG. Clinical trials update: RENEWAL (RENAISSANCE and RECOVER) and ATTACH. Eur J Heart Fail 2002;4:559-61.
[Google Scholar]
|
| 60. |
Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack M. Identification of a novel role for sphingolipid signaling in TNF-alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol 2002;34:509-18.
[Google Scholar]
|
| 61. |
Westlake SL, Colebatch AN, Baird J, Curzen N, Kiely P, Quinn M, et al. Tumour necrosis factor antagonists and the risk of cardiovascular disease in patients with rheumatoid arthritis: A systematic literature review. Rheumatology 2011;50:518-31.
[Google Scholar]
|
| 62. |
Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990;323:236-41.
[Google Scholar]
|
| 63. |
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275-85.
[Google Scholar]
|
| 64. |
Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: A systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol 2011;64:1035-50.
[Google Scholar]
|
| 65. |
Gelfand JM, Berlin J, Van Voorhees A, Margolis DJ. Lymphoma rates are low but increased in patients with psoriasis: Results from a population-based cohort study in the United Kingdom. Arch Dermatol 2003;139:1425-9.
[Google Scholar]
|
| 66. |
Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2007;44:265-7.
[Google Scholar]
|
| 67. |
Geborek P, Bladstrom A, Turesson C, Gulfe A, Petersson IF, Saxne T, et al. Tumour necrosis factor blockers do not increase overall tumour risk in patients with rheumatoid arthritis, but may be associated with an increased risk of lymphomas. Ann Rheum Dis 2005;64:699-703.
[Google Scholar]
|
| 68. |
Levine D, Strober BE. The treatment of moderate-to-severe psoriasis: Prescreening and monitoring psoriatic patients on biologics. Semin Cutan Med Surg 2010;29:28-34.
[Google Scholar]
|
| 69. |
Andres E, Dali-Youcef N, Serraj K, Zimmer J. Recognition and management of drug-induced cytopenias: The example of idiosyncratic drug-induced thrombocytopenia. Expert Opin Drug Saf 2009;8:183-90.
[Google Scholar]
|
| 70. |
Bessissow T, Renard M, Hoffman I, Vermeire S, Rutgeerts P, Van Assche G. Review article: Non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther 2012;36:312-23.
[Google Scholar]
|
| 71. |
Ferraccioli G, Gremese E. Thrombogenicity of TNF alpha in rheumatoid arthritis defined through biological probes: TNF alpha blockers. Autoimmun Rev 2004;3:261-6.
[Google Scholar]
|
| 72. |
Jonsdottir T, Bratt J, Klareskog L, Van Vollenhoven R. Development of ACLA (anti-cardiolipin antibodies) in patients treated with infliximab (remicade). Arthritis Rheum 2001;44 Suppl:S373.
[Google Scholar]
|
| 73. |
Marchesoni A, Arreghini M, Panni B, Battafarano N, Uziel L. Life-threatening reversible bone marrow toxicity in a rheumatoid arthritis patient switched from leflunomide to infliximab. Rheumatology (Oxford) 2003;42:193-4.
[Google Scholar]
|
| 74. |
Cancelliere N, Barranco P, Vidaurrázaga C, Benito DM, Quirce S. Subacute prurigo and eosinophilia in a patient with rheumatoid arthritis receiving infliximab and etanercept. J Investig Allergol Clin Immunol 2011;21:248-9.
[Google Scholar]
|
| 75. |
Metyas SK, Tadros RM, Arkfeld DG. Adalimumab-induced noncaseating granuloma in the bone marrow of a patient being treated for rheumatoid arthritis. Rheumatol Int 2009;29:437-9.
[Google Scholar]
|
| 76. |
Vermeire S, Noman M, Van Assche G, Baert F, Van Steen K, Esters N, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn's disease: A prospective cohort study. Gastroenterology 2003;125:32-9.
[Google Scholar]
|
| 77. |
Ramanan AV, Schneider R. Macrophage activation syndrome following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol 2003;30:401-3.
[Google Scholar]
|
| 78. |
Kinder AJ, Edwards J, Samanta A, Nichol F. Pregnancy in a rheumatoid arthritis patient on infliximab and methotrexate. Rheumatology (Oxford) 2004;43:1195-6.
[Google Scholar]
|
| 79. |
Srinivasan R. Infliximab treatment and pregnancy outcome in active Crohn's disease. Am J Gastroenterol 2001;96:2274-5.
[Google Scholar]
|
| 80. |
Mahadevan U, Kane S, Sandborn WJ, Cohen RD, Hanson K, Terdiman JP, et al. Intentional infliximab use during pregnancy for induction or maintenance of remission in Crohn's disease. Aliment Pharmacol Ther 2005;21:733-8.
[Google Scholar]
|
| 81. |
Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB. A safety assessment of tumor necrosis factor antagonists during pregnancy: A review of the Food and Drug Administration database. J Rheumatol 2009;36:635-41.
[Google Scholar]
|
| 82. |
Stengel JZ, Arnold HL. Is infliximab safe to use while breastfeeding? World J Gastroenterol 2008;14:3085-7.
[Google Scholar]
|
| 83. |
Ostensen M, Eigenmann GO. Etanercept in breast milk. J Rheumatol 2004;31:1017-8.
[Google Scholar]
|
| 84. |
Bachmann F, Nast A, Sterry W, Philipp S. Safety and efficacy of thetumor necrosis factor antagonists. Semin Cutan Med Surg 2010;29:35-47.
[Google Scholar]
|
Fulltext Views
14,341
PDF downloads
3,385





