Translate this page into:
Varenicline-induced symmetrical drug-related intertriginous and flexural exanthema
2 Department of Dermatology, Drug Hypersensitivity Clinical and Research Center, Chang Gung Memorial Hospital; Chang Gung Immunology Consortium, Chang Gung Memorial Hospital, Chang Gung University, Taoyuan, Taiwan
3 Department of Dermatology, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taoyuan, Taiwan
4 Department of Dermatology, Drug Hypersensitivity Clinical and Research Center, Chang Gung Memorial Hospital; Chang Gung Immunology Consortium, Chang Gung Memorial Hospital, Chang Gung University; College of Medicine, Chang Gung University, Taoyuan; Whole-Genome Research Core Laboratory of Human Diseases, Chang Gung Memorial Hospital, Keelung, Taiwan
Correspondence Address:
Wen-Hung Chung
Chang Gung Memorial Hospital, 199, Tun-Hwa North Road, Taipei 105
Taiwan
| How to cite this article: Cheng CY, Wang CW, Wang FY, Chung WH. Varenicline-induced symmetrical drug-related intertriginous and flexural exanthema. Indian J Dermatol Venereol Leprol 2019;85:209-211 |
Sir,
Symmetrical drug-related intertriginous and flexural exanthema is a drug-induced eruption characterized by symmetrical distribution of well-demarcated erythema of the inguinal, gluteal and other intertriginous areas. The most common offending drug is β-lactam antibiotics, particularly amoxicillin. In addition, acetaminophen, hydroxyzine, ranitidine and radiocontrast have been reported to be causative drugs.[1],[2] Varenicline is a selective partial agonist for the α4β2 nicotinic acetylcholine receptor, which was approved by the United States Food and Drug Administration in May 2006 for tobacco cessation. We were unable to find any previous reports of varenicline-induced symmetrical drug-related intertriginous and flexural exanthema. Here, we report a rare case of varenicline-induced symmetrical drug-related intertriginous and flexural exanthema.
A 50-year-old male presented to our clinic with progressive itchy skin rash over neck and buttocks which had persisted for 20 days. These skin lesions had developed 2 days after taking varenicline 1.0 mg daily for smoking cessation. The patient reported a history of epilepsy, treated with oxcarbazepine for the past 2 years without dose adjustment in the last year. On physical examination, erythematous macules and patches were found to be symmetrically distributed over the buttocks, anterior neck and inguinal region [Figure - 1]a and [Figure - 1]b. There was no mucosal involvement, blister or pustule formation. Systemic examination was within normal limits. Laboratory tests revealed no other systemic involvement. A skin biopsy from the buttock region showed basket-weave hyperkeratosis, mild acanthosis, perivascular lymphocytes and eosinophil infiltration [Figure - 2].
 |
| Figure 1: |
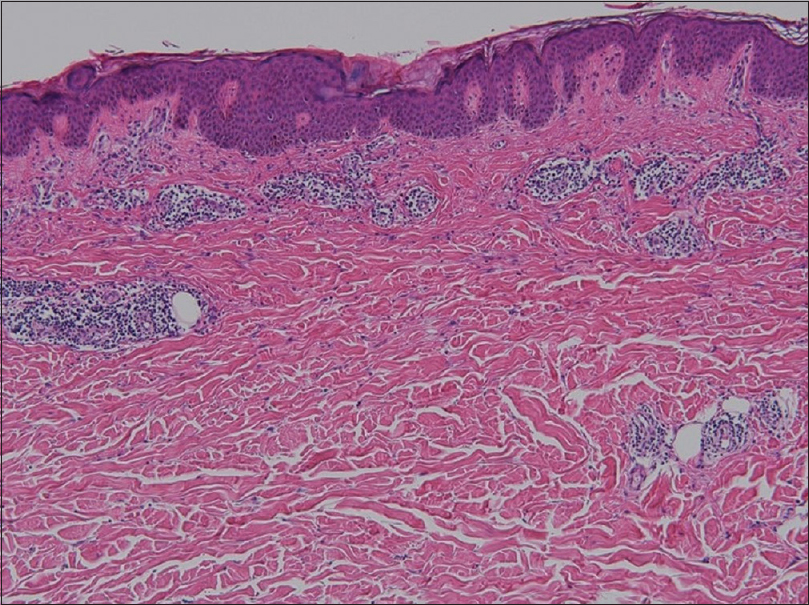 |
| Figure 2: Histopathology showed basket wave hyperkeratosis, mild acanthosis and perivascular lymphocytes and eosinophils infiltration (hematoxylin and eosin, ×100) |
On the basis of these findings, symmetrical drug-related intertriginous and flexural exanthema was diagnosed. The causality of varenicline-induced symmetrical drug-related intertriginous and flexural exanthema was in the probable category according to the Naranjo Adverse Drug Reaction Probability Scale (a score of 6) [Table - 1].[3] Administration of varenicline was discontinued, and the patient received one dose of 5 mg dexamethasone intramuscular injection. Oral antihistamine and mometasone furoate 0.1% cream were also prescribed. After treatment for 2 weeks, the skin lesions gradually resolved. One month later, we performed lymphocyte activation assay by measuring granulysin level which has been previously published.[4] Drugs were diluted in the medium to obtain a concentration reflecting the physiological therapeutic range. The result revealed a positive finding of 1.7-fold elevation in granulysin level when varenicline was administered compared with a negative control of phosphate buffered saline (normal healthy control, 1.04 ± 0.24-fold elevation in granulysin level). Oxcarbazepine findings were negative. Oral provocation test was not performed because varenicline could lower seizure threshold. The patient was followed up for 3 months; with no recurrence.

The term SDRIFE that is symmetrical drug-related intertriginous and flexural exanthema was first described by Häusermann et al. in 2004, and five diagnostic criteria were proposed: (1) Exposure to a systemically administered drug either at the first or repeated dose (excluding contact allergens); (2) sharply demarcated erythema of the gluteal/perianal area and/or V-shaped erythema of the inguinal/perigenital area; (3) involvement of at least one other intertriginous/flexural localization; (4) symmetry of affected areas; and (5) absence of systemic symptoms and signs.[5] Our case met all the above-mentioned criteria.
The diagnosis of symmetrical drug-related intertriginous and flexural exanthema may be easily missed, and it should be differentiated from other flexural eruptions, such as candidal intertrigo, inverse psoriasis or acute generalized exanthematous pustulosis. Besides, symmetrical drug-related intertriginous and flexural exanthema should also be differentiated from systemic contact dermatitis. Symmetrical drug-related intertriginous and flexural exanthema is induced by systemic medication without previous cutaneous sensitization while systemic contact dermatitis is caused by systemic exposure of previous sensitized contact allergen or a cross-reacting molecule. Thorough medical history taking is important for early diagnosis. Symmetrical drug-related intertriginous and flexural exanthema is classified as a type IV hypersensitivity reaction. The skin rash often develops hours to days after exposure to the causative drugs. The histopathology of symmetrical drug-related intertriginous and flexural exanthema is nonspecific and frequently reveals superficial perivascular lymphocytic infiltration with the occasional presentation of neutrophils or eosinophils. Symmetrical drug-related intertriginous and flexural exanthema is diagnosed mainly on history and clinical manifestations. Other diagnostic tests include patch test, lymphocyte transformation test and provocation test. Although patch test is a common method for identifying the causative drug in symmetrical drug-related intertriginous and flexural exanthema, only half of the cases show a positive result.[5] The lymphocyte transformation test is an in vitro examination which measures the proliferation of T cells in the presence of a specific antigen; however, low sensitivity limits its diagnostic value. Granulysin is a cytolytic molecule which is primarily expressed in the natural killer cells and cytotoxic T cells. In the past decade, Chung et al. demonstrated that granulysin is the key cytotoxic mediator of Stevens–Johnson syndrome and toxic epidermal necrolysis.[6] In addition, Schlapbach et al. found that granulysin could be induced in vitro in patients with several kinds of cutaneous adverse reaction in addition to Stevens–Johnson syndrome and toxic epidermal necrolysis, such as maculopapular eruption, acute generalized exanthematous pustulosis or fixed drug eruption.[7] The present case report demonstrates that granulysin can be induced by the causative drug in patients with symmetrical drug-related intertriginous and flexural exanthema. The result indicates that granulysin may play a role in the pathogenesis of symmetrical drug-related intertriginous and flexural exanthema, and in vitro granulysin assay may be a useful diagnostic tool. However, further well-controlled study is warranted for its clinical application in the diagnosis of symmetrical drug-related intertriginous and flexural exanthema.
In conclusion, we report a rare case of varenicline-induced symmetrical drug-related intertriginous and flexural exanthema; the causality was confirmed by in vitro granulysin assay. Physicians should be aware of this potential adverse cutaneous reaction in patients receiving varenicline for smoking cessation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Akkari H, Belhadjali H, Youssef M, Mokni S, Zili J. Baboon syndrome induced by hydroxyzine. Indian J Dermatol 2013;58:244.
[Google Scholar]
|
| 2. |
Binitha MP, Sasidharanpillai S, John R, Sherjeena PV. Symmetrical drug-related intertriginous and flexural exanthema due to ranitidine. Indian J Pharmacol 2014;46:551-2.
[Google Scholar]
|
| 3. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
| 4. |
Chung WH, Pan RY, Chu MT, Chin SW, Huang YL, Wang WC, et al. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol 2015;135:2237-48.
[Google Scholar]
|
| 5. |
Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: Is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 2004;51:297-310.
[Google Scholar]
|
| 6. |
Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343-50.
[Google Scholar]
|
| 7. |
Schlapbach C, Zawodniak A, Irla N, Adam J, Hunger RE, Yerly D, et al. NKp46+ cells express granulysin in multiple cutaneous adverse drug reactions. Allergy 2011;66:1469-76.
[Google Scholar]
|
Fulltext Views
3,145
PDF downloads
2,217





