Translate this page into:
Can a test-dose of methotrexate cause methotrexate-induced epidermal necrosis?
2 Department of Dermatology; Department of Pathology, Porto Alegre Clinical Hospital, Porto Alegre, Brazil
3 Department of Faculty of Medicine, University of Rio Grande do Sul, Porto Alegre, Brazil
Correspondence Address:
Gabriela Fortes Escobar
Department of Dermatology, Hospital de Clínicas de Porto Alegre, Rua Ramiro Barcelos, 2350, Porto Alegre, RS 90035-903
Brazil
| How to cite this article: Escobar GF, Cartell A, Ribeiro CK, Bastiani MD. Can a test-dose of methotrexate cause methotrexate-induced epidermal necrosis?. Indian J Dermatol Venereol Leprol 2020;86:70-72 |
Sir,
The development of methotrexate-induced epidermal necrosis is a rare adverse reaction to this drug, usually associated with chronic kidney disease and high initial dosage.[1] However, in this report, we highlight that methotrexate-induced epidermal necrosis may occur only after a test-dose, even in the absence of a significant renal impairment.
A 54-year-old hypertensive and diabetic woman was referred to our Department with palmoplantar pustular psoriasis. She had been using furosemide, propranolol, metformin, and acetylsalicylic acid regularly for 2 years. The patient failed to respond to several previous psoriasis treatments, including topical preparations (potent steroids and coal tar), colchicine, and dapsone. A new treatment attempt with methotrexate was suggested, as soon as a thorough pre-evaluation was performed and showed no contraindication. Pretreatment routine biochemistry was unremarkable except for mild renal dysfunction [creatinine: 0.9 mg/dL and estimated glomerular filtration rate (eGFR): 73 mL/min]. Thus, a test-dose of subcutaneous methotrexate was administred (7.5 mg/week) [day 0] followed by oral folic acid (5 mg) 2 days later. On day 4 the patient presented with a bullous eruption. Examination revealed hemorrhagic bullae on the palms and soles [Figure - 1], along with few scattered lesions on the lower extremities and thorax. Mucosal involvement of the upper and lower lips could be observed, showing extensive irregular ulcerations, cracking, and fissuring with blood encrustation [Figure - 2]. Intraoral and genital ulcerations were also visualized. Immediately after the first assessment, methotrexate was suspended. Histopathological examination of a lesion on the leg revealed a vacuolar interface dermatitis with epidermal necrosis, dyskeratosis, and moderate inflammatory infiltrate with eosinophils [Figure - 3]. Laboratory investigations detected neutropenia (0.42 × 109/L; normal value: 1.50–7.00 × 109/L), leukopenia (2.51 × 109/L; normal value: 3.60–11.00 × 109/L), thrombocytopenia (69 × 109/L; normal value: 150–400 × 109/L), and elevated aminotransferases [alanine aminotransferase (ALT): 219 U/L; normal value 10–34 U/L and aspartate aminotransferase (AST): 136 U/L; normal value: 10–49 U/L]. Based on the history, clinical examination, and histopathologic findings, a final diagnosis of methotrexate-induced epidermal necrosis was established, associated with myelosuppression and hepatic injury. After 10 days of drug withdrawal, cutaneous lesions resolved with re-epithelization, along with progressive normalization of laboratory parameters.
 |
| Figure 1: Hemorrhagic bullae on the palmar region |
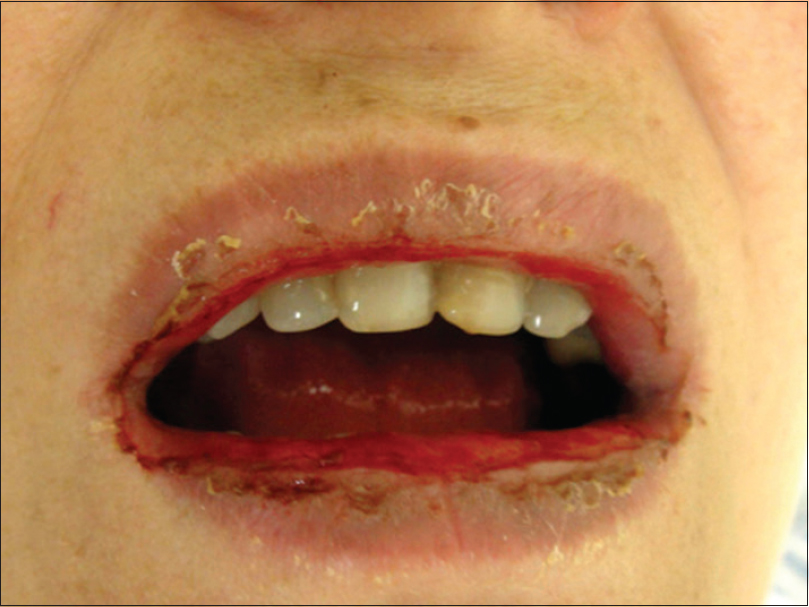 |
| Figure 2: Extensive irregular ulcerations, cracking, and fissuring with hemorrhagic crusting of the mucosal upper and lower lips |
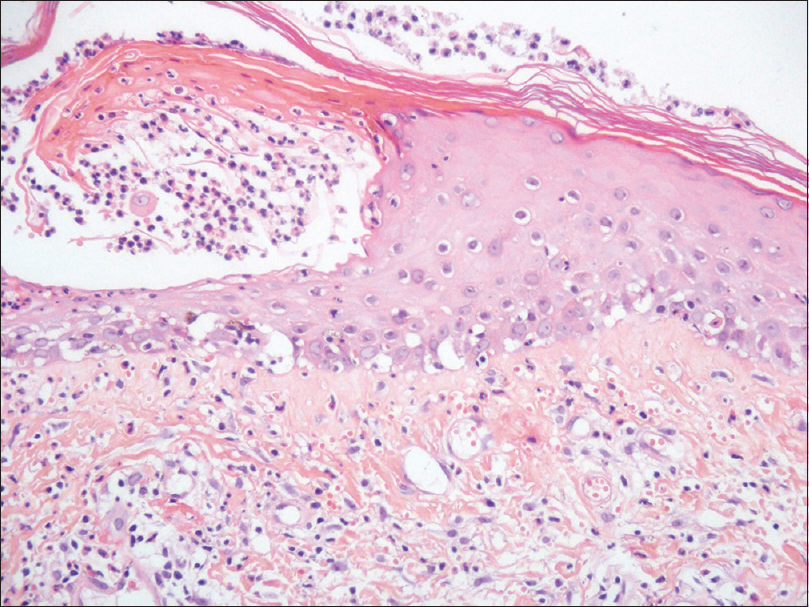 |
| Figure 3: Vacuolar interface dermatitis with epidermal necrosis, dyskeratosis, and chronic inflammatory infiltrate with eosinophils (H and E, ×200) |
Methotrexate-induced epidermal necrosis is a rare acute idiosyncratic reaction that may be life-threating.[2] It is characterized clinically by skin detachment and mucosal erosions, which mimics Stevens–Johnsons syndrome and toxic epidermal necrolysis. Therefore, reports have previously described cases as “methotrexate-induced necrolysis”[2] or “pseudo-toxic epidermal necrolysis”.[3] Recently, a case series of 24 patients has established the term methotrexate-induced epidermal necrosis.[1] This study compared cases with a control group of 150 patients who were also using methotrexate. The most important risk factors for methotrexate-induced epidermal necrosis included older age (>60 years), chronic renal disease (eGFR < 60 mL/min), and a high initial dosage (>10 mg/week) without folic acid supplementation. Interestingly, our patient did not present any of the risk factors: she was younger than the cutoff age, she did not present with a significant decrease in renal function, the initial dose of methotrexate was very low (test-dose), and it was followed by supplementation with folic acid.
In the same case-series variable degree of skin detachment was observed, ranging from 8% to 80% of total body surface area.[1] In addition, approximately half of the patients had leukopenia and thrombocytopenia, while significant hepatic impairment was present in only 1 case (AST 184 U/L and ALT 183 U/L). The median duration of methotrexate exposure was 34 days (ranging from 3 to 90 days), with a median initial dose of 12.4 mg. Additional findings included concomitant nonsteroidal anti-inflammatory drug use [20.8%(n = 5)] and hypoalbuminemia (<3.5 g/dL) [85.7% (n = 18)] in methotrexate-induced epidermal necrosis cases. Our patient presented with a small area of skin detachment (8%), along with leukopenia and thrombocytopenia. Although uncommon, our case also presented with elevated hepatic enzymes, adding to the single case described by Chen et al.[1] Remarkably, our patient developed symptoms within 4 days of a test-dose of 7.5 mg; however, she was using concomitant acetylsalicylic acid, which may have increased free active methotrexate in the serum, as both drugs compete for albumin-binding sites.[4],[5] Furthermore, our patient had a borderline albumin level (3.5 g/dL).
Since methotrexate is used in a wide spectrum of dermatologic diseases, we believe it is important to report a significant cutaneous reaction to this drug, to raise awareness about this possible event.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Chen TJ, Chung WH, Chen CB, Hui RC, Huang YH, Lu YT, et al. Methotrexate-induced epidermal necrosis: A case series of 24 patients. J Am Acad Dermatol 2017;77:247-55.
[Google Scholar]
|
| 2. |
Reed KM, Sober AJ. Methotrexate-induced necrolysis. J Am Acad Dermatol 1983;8:677-9.
[Google Scholar]
|
| 3. |
Sawada Y, Kawakami C, Nakamura M, Tokura Y, Yoshiki R. Toxic epidermal necrosis-like dermatosis induced by the first course of methotrexate. Eur J Dermatol 2009;19:397-8.
[Google Scholar]
|
| 4. |
Romão VC, Lima A, Bernardes M, Canhão H, Fonseca JE. Three decades of low-dose methotrexate in rheumatoid arthritis: Can we predict toxicity? Immunol Res 2014;60:289-310.
[Google Scholar]
|
| 5. |
Yélamos O, Català A, Vilarrasa E, Roé E, Puig L. Acute severe methotrexate toxicity in patients with psoriasis: A case series and discussion. Dermatology 2014;229:306-9.
[Google Scholar]
|
Fulltext Views
4,575
PDF downloads
2,659





