Translate this page into:
Fixed drug eruption due to modafinil
2 Department of Dermatology and STD, UCMS and GTB Hospital, Delhi, India
3 MAMC and L N Hospital, New Delhi, India
4 Department of Psychiatry, Westmead Hospital, New South Wales, Australia
Correspondence Address:
Sidharth Sonthalia
Skinnocence: The Skin Clinic, C-2246, Sushant Lok-1, Block-C, Gurgaon - 122 009
India
| How to cite this article: Sonthalia S, Arora R, Sarkar R, Dhawan A, Srivastava A. Fixed drug eruption due to modafinil. Indian J Dermatol Venereol Leprol 2014;80:90-92 |
Sir,
Modafinil is a relatively novel wakefulness-promoting agent, used to treat narcolepsy, shift-work sleep disorder (SWD), and obstructive sleep apnea (OSA). [1] Unlike older psychostimulants, the relative lack of side effects like anxiety have made modafinil popular, especially among business process outsource (BPO) professionals and sports personnel. Apart from neuropsychiatric and other systemic adverse effects, modafinil is known to result in various cutaneous reactions. [2] However, to the best of our knowledge, only one case of modafinil-induced fixed drug eruption (FDE) has been reported to date. [3]
A 32-year-old BPO professional presented with mildly pruritic, multiple, round-to-oval, edematous, well-circumscribed plaques of size 1 × 2 cm to 10 × 10 cm over the anterior abdomen, lower back, and elbows, with a central dusky-red to violaceous hue [Figure - 1]. The oral and genital mucosae were spared. The patient had taken a single dose of modafinil (100 mg) 12 hours preceding the eruption, without prescription, to enhance his work efficiency for a night-shift. He gave no past history of taking modafinil or any other drug. The general physical and systemic examinations were unremarkable. Histopathology from a buttock lesion revealed hyperkeratosis with vacuolar changes at the dermoepidermal junction and an upper dermal perivascular lymphocytic infiltrate with eosinophils, suggestive of FDE.
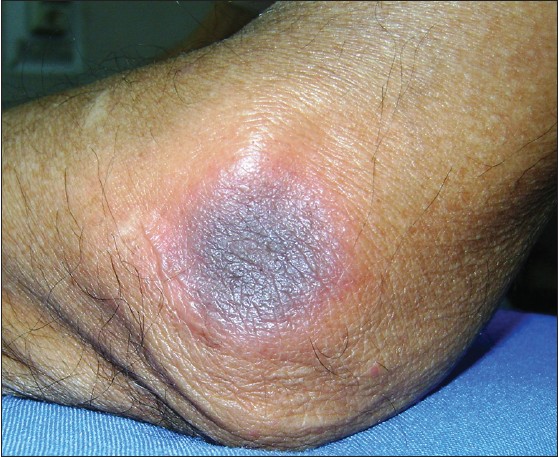 |
| Figure 1: Circumscribed erythematous plaque with central dusky red to violaceous hue over the elbow |
The patient was advised to stop taking modafinil. Oral anti-histaminics and topical mometasone resulted in the subsidence of the lesions within two weeks, leaving residual post-inflammatory hyperpigmentation (PIH). With the patient′s consent, an oral provocation test (OPT) was performed after four weeks of resolution of the lesions with 25 mg modafinil (one-fourth dose). Lesional flare-up occurred within 12 hours, but subsided with two weeks of topical steroids, confirming modafinil to be the causative drug of FDE.
Fixed drug eruption is a common adverse drug reaction (ADR) characterized by a sudden onset of edematous and erythematous to violaceous plaques, with sharply demarcated borders. The common offending drugs include sulfonamides, non-steroidal anti-inflammatory drugs, phenazones, and tetracyclines. Although, histopathology and drug patch testing aid in the confirmation of diagnosis, OPT is considered the diagnostic gold standard.
Modafinil is available in the form of 100 or 200 mg tablets. Owing to its additional mood-brightening and fatigue-relieving effects, modafinil has also been used therapeutically for conditions like disease-related fatigue, attention-deficit disorder, Alzheimer′s disease, depression, cognitive impairment, and de-addiction from other psychostimulants. [1] Its pharmacological profile, which is notably different from the traditional psychostimulants like cocaine or methylphenidate, makes adverse effects like excess locomotor activity, anxiety or jitteriness rare. However, various neuropsychiatric, cardiovascular, and gastrointestinal side effects have been reported. [4] The cutaneous reactions to modafinil reported to date range from mild reactions like pruritus, urticaria, scaling, oral ulcers, and the like, to serious reactions like angioedema, anaphylaxis, erythema multiforme (EM), Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and the Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome. [2],[5] From the date of initial marketing until now, the Food and Drug administration (FDA) has received six cases of severe cutaneous ADRs associated with this drug. [5] Estimates of the background incidence rates of serious skin reactions range from one to two cases per million years. Typically the eruption occurs within the first five weeks of treatment, although it may appear as late as three months after treatment. [5]
To the best of our knowledge, modafinil-induced FDE has been reported only once before, by Gaikwad and Dhuri. [3] Their patient was a student who developed FDE within 12 hours of taking 200 mg of modafinil, prescribed by a psychiatrist, to improve wakefulness for a competitive examination. The eruption in his case was localized only to the lips. In contrast, our patient had multifocal lesions, with complete sparing of the mucosae. Furthermore, Gaikwad and Dhuri considered modafinil as the causative agent for this ADR based on a Naranjo scale of 5 (questionnaire to determine the likelihood of a drug as a cause of an ADR), unlike the OPT done in our case. [3] FDE has been reported with other psychostimulants like cocaine and methylphenidate. [6] However, this is the first case report of modafinil-induced, multifocal FDE.
The mood elevating and memory-enhancing effects of modafinil are resulting in the abuse of modafinil as a lifestyle drug, especially among students and BPO professionals, who conveniently procure it as a non-prescription drug. India, being the global hub for BPO, the potential of modafinil abuse is alarming. A recent report has confirmed its increasing use by medical professionals as well. [7] Thus, all dermatologists and neuropsychiatrists should be aware of the prevalent misuse of this drug and the wide spectrum of its cutaneous and other complications.
| 1. |
Kim D. Practical use and risk of modafinil, a novel waking drug. Environ Health Toxicol 2012;27:e2012007.
[Google Scholar]
|
| 2. |
Modafinil: Serious skin reactions. Prescrire Int 2007;16:71.
[Google Scholar]
|
| 3. |
Gaikwad GV, Dhuri CV. Modafinil-induced Fixed Drug Eruption. Indian J Psychol Med 2012;34:383-4.
[Google Scholar]
|
| 4. |
Provigil Side Effects Center. Available from: http://www.rxlist.com/provigil-side-effects-drug-center.htm. [Last accessed on 2013 May 8].
[Google Scholar]
|
| 5. |
FDA Drug Safety Newsletter. Modafinil (marketed as Provigil): Serious skin reactions. Available from: http://www.fda.gov/Drugs/DrugSafety/DrugSafetyNewsletter/ucm115974.htm. [Last accessed on 2013 May 8, and updated on 2010 Apr 10].
[Google Scholar]
|
| 6. |
Cohen HA, Ashkenazi A, Nussinovitch M, Gross S, Frydman M. Fixed drug eruption of the scrotum due to methylphenidate. Ann Pharmacother 1992;26:1378-9.
[Google Scholar]
|
| 7. |
McBeth BD, McNamara RM, Ankel FK, Mason EJ, Ling LJ, Flottemesch TJ, et al. Modafinil and zolpidem use by emergency medicine residents. Acad Emerg Med 2009;16:1311-7.
[Google Scholar]
|
Fulltext Views
5,280
PDF downloads
3,698





