Translate this page into:
Diagnostic role of dermatoscopy in porokeratotic adnexal ostial nevus
2 Department of Dermatology, R. G. Kar Medical College, Kolkata, West Bengal, India
Correspondence Address:
Nilendu Sarma
P N Colony, Sapuipara, Bally, Howrah, West Bengal
India
| How to cite this article: Sarma N, Chakraborty S. Diagnostic role of dermatoscopy in porokeratotic adnexal ostial nevus. Indian J Dermatol Venereol Leprol 2019;85:546-548 |
Sir,
Porokeratotic adnexal ostial nevus is a rare, relatively newly described variant of porokeratotic eccrine hamartoma. It was first reported in 1979 by Marsden et al.,[1] although the terminology was subsequently coined by Abell and Read.[2]
Cases where invaginated cornoid lamella involves dilated eccrine duct are termed as porokeratotic eccrine ostial and dermal duct nevus. When such lamella involves hair follicle, it is known as porokeratotic eccrine and hair follicle nevus. It has been proposed that most cases involve both follicle and eccrine ducts and true distinction between these entities does not exist. Thus, porokeratotic adnexal ostial nevus is considered a more unifying term for these conditions.[3]
A 23-year-old woman presented with multiple dark, asymptomatic, firm linear eruptions on her right leg since early childhood. After initial progression, it became stable. No other members of the family had a similar condition.
The eruptions were found to be firm, dark papular eruptions, distributed along Blaschko's lines on the right lower limb. These involved dorsum of right foot, anterior leg and extended up to upper thigh.
Papules were discrete on the foot, but on the leg and thigh multiple papules grouped to form larger well-defined plaques. Individual papules could not be dislodged with a firm sliding force. Marginal crater with invagination was noticed in some lesions [Figure - 1].
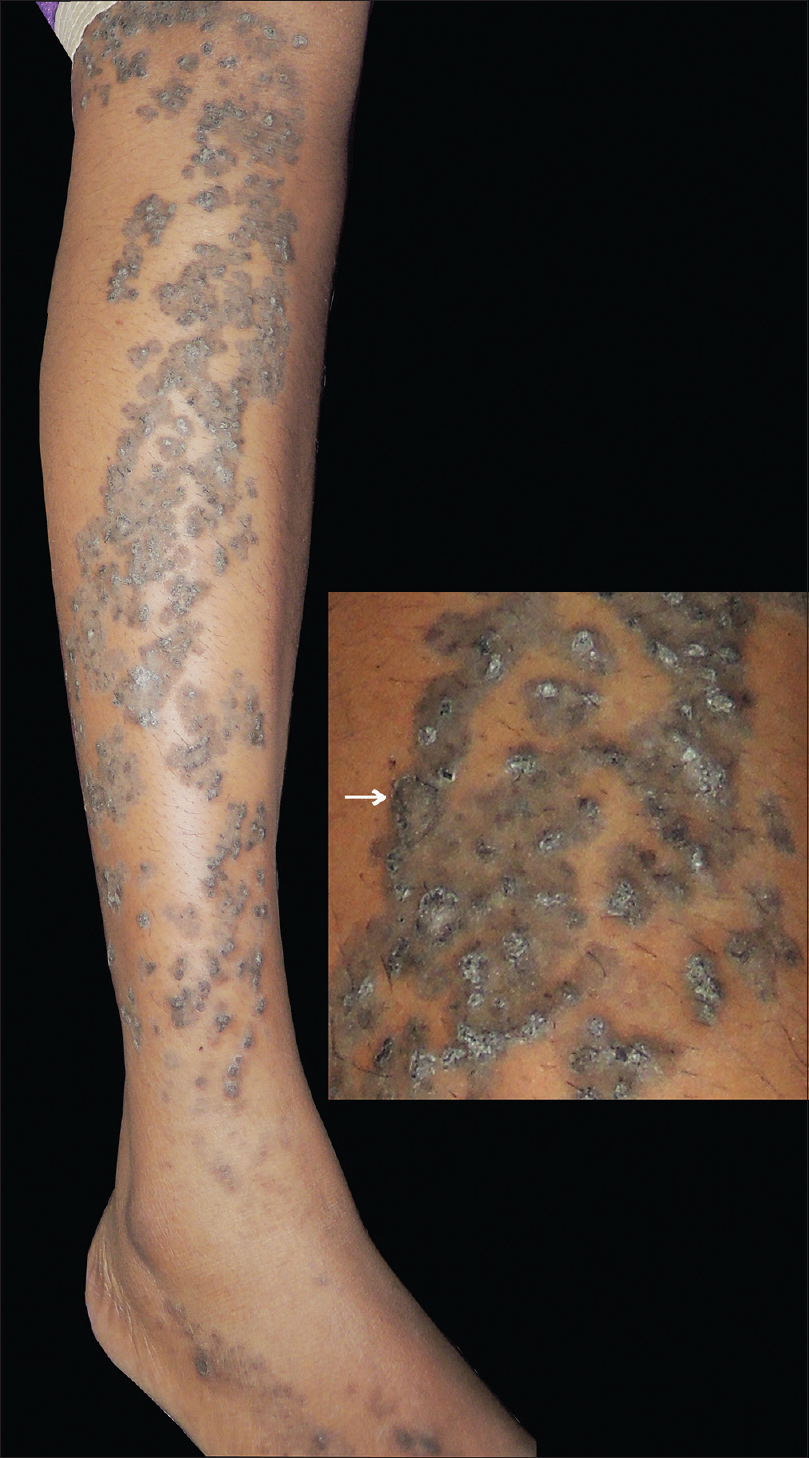 |
| Figure 1: Grouped dark papules of porokeratotic adnexal ostial nevus distributed along Blaschko's line on right leg in a young lady. Inset: close-up view showing grouped papules forming plaque, and some papules having the invagination at the margin (arrow) |
Dermatoscopic examination was done with DermLite 4™. A typical hyperkeratotic circinate margin that outlined the porokeratosis appeared as well-defined dark-colored rim under dermatoscopy. Prominent whitish areas were detected to surround the rim and possibly indicated the aberrant keratinization. Keratin plugs that appeared as white milia-like structures were detected.
This bluish gray background color extended much beyond the rim. Although this was visible clinically with naked eyes, it was appreciated much clearly under dermatoscope. It possibly indicated dermal melanophages that were confirmed histologically. This clearly indicated the actual extent of the lesion and there were numerous, homogenously distributed tiny white dots, most possibly representing the opening of the eccrine ducts. Such accentuated white dots were not found in the surrounding normal areas. Widened follicular openings were also found [Figure - 2].
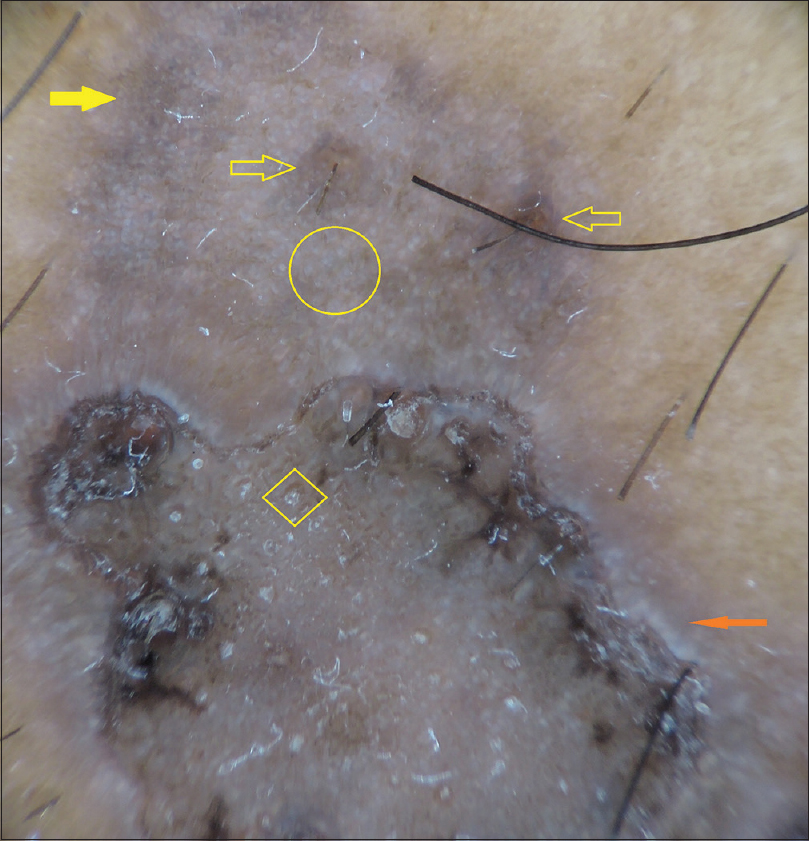 |
| Figure 2: Dermoscopy (DermLite 4, ×10, polarized mode) shows well-defined irregular dark-colored rim encircling the main lesion. A prominent whitish band (solid red arrow) surrounds the darker rim. Within the encircled area, there are many prominent milia-like white structures (within a diamond) that possibly represent follicular plugs. Numerous homogenously distributed white tiny dots (circle) represent eccrine ducts. Eccrine ducts spared follicular openings that appeared large and wide (arrow without fill). The lesional margin is formed by a dark bluish gray veil (solid yellow arrow) |
Histopathological examination revealed hyperkeratosis and acanthosis. There was a prominent invaginated thick keratin plug with cornoid lamella (parakeratotic column) with underlying multiple dermal eccrine ducts. One hair follicle was also noted below the cornoid lamella [Figure - 3]. The area just beneath the cornoid lamella showed the absent granular layer. Dyskeratotic cells were also detected [Figure - 4]. There were prominent dermal melanophages and a perivascular and periappendageal inflammatory infiltration.
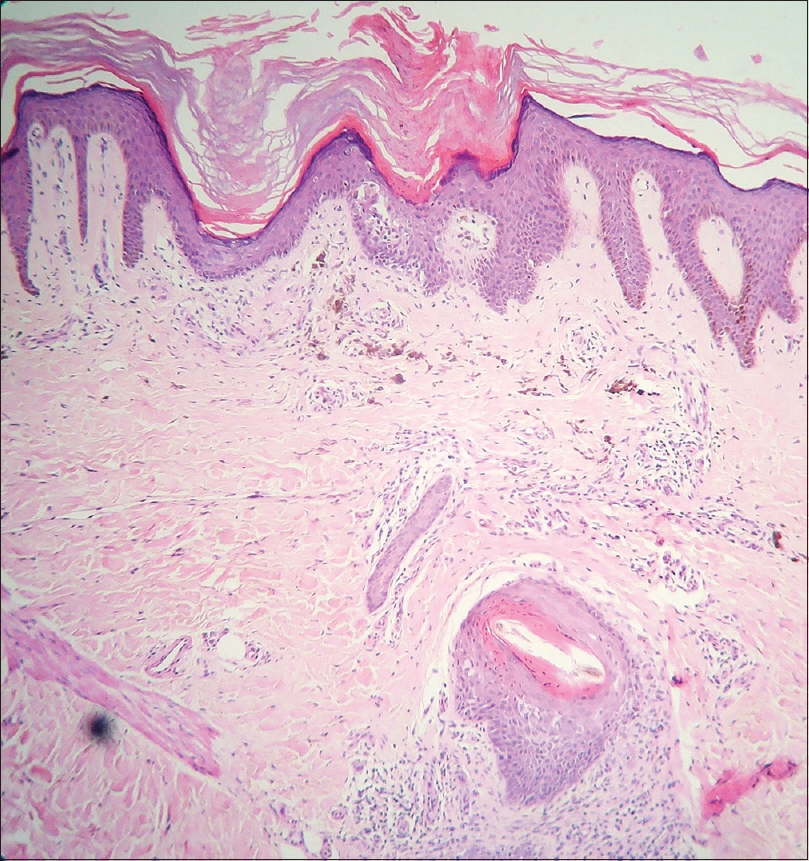 |
| Figure 3: Focal hyperkeratosis, acanthosis and keratin plug invaginating within dermis closely associated with numerous, multiple eccrine ducts in close association with coronoid lamella, and hypogranulosis. A hair follicle lies deep into the dermis in the same vertical plane. Mild mixed inflammatory cells were also found. Dermal melanophages and inflammatory infiltrate were also noted (hematoxylin and eosin, ×100) |
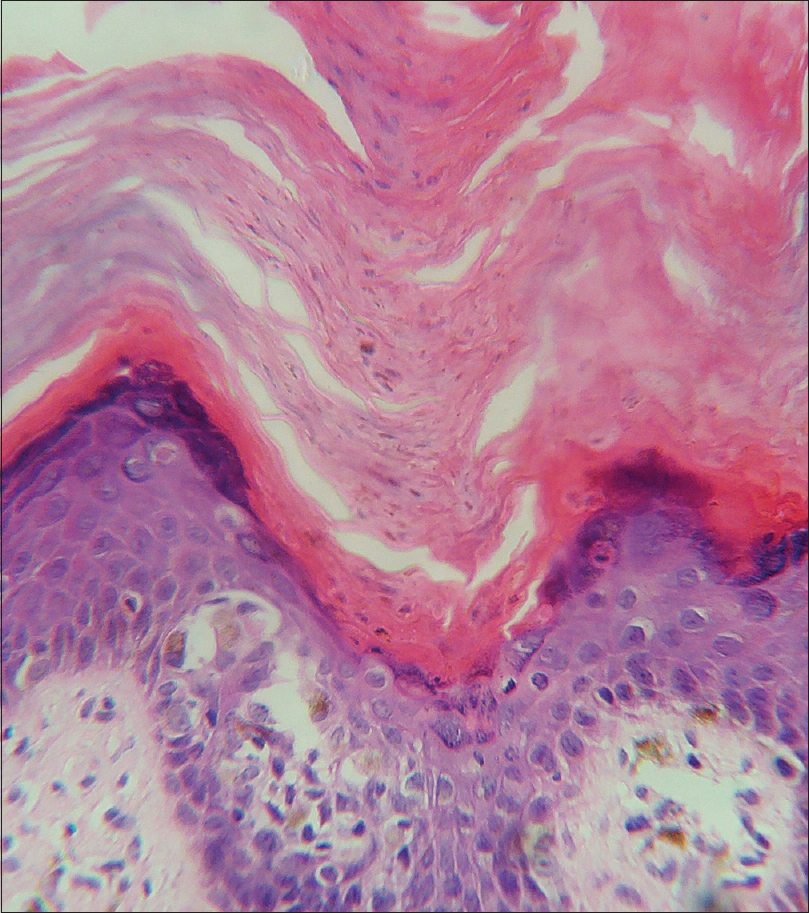 |
| Figure 4: Higher magnification shows a prominent parakeratotic column, absent granular layer and dyskeratotic cells (hematoxylin and eosin, ×400 |
Histopathological findings confirmed the diagnosis of porokeratotic adnexal ostial nevus or porokeratotic eccrine ostial and dermal duct nevus. The patient was treated with topical keratolytic such as salicylic acid 6% ointment, 10% urea and topical tazarotene gel (0.1%) without any benefit.
Porokeratotic adnexal ostial nevus is generally present at birth. This is characterized by the presence of asymptomatic areas of plugged pits, spiny keratotic filiform papules which may also enlarge to papules, nodules and plaque. This may be localized, generalized showing a segmental or Blaschkoid linear distribution.
It has been proposed that single GJB2 somatic mutation could cause porokeratotic eccrine ostial and dermal duct nevus.[4] KID syndrome could be a more generalized form of porokeratotic eccrine ostial and dermal duct nevus. Both these conditions share many similarities in histopathology and molecular and genetic peculiarity.[5]
Cornoid lamella is considered to represent the abnormal clone of cells that may actually originate in the eccrine ducts or that may result from focal abnormal keratinization of the epidermis due to mosaicism.
Porokeratotic adnexal ostial nevus needs to be differentiated from linear porokeratosis, focal dermal hypoplasia, inflammatory linear verrucous epidermal nevus, linear psoriasis, linear Darier's disease, linear hypertrophic lichen planus and curvilinear ichthyosis in Conradi- Hünermann syndrome, CHILD syndrome (Congenital hemidysplasia with ichthyosiform erythroderma and limb defects) and nevus comedonicus.
Linear porokeratosis is unassociated with dilated and hyperplastic eccrine ostia and/or hair follicles. It also lacks hypogranulosis.
Dilated and hypertrophic eccrine ducts and follicles are considered key alterations in porokeratotic adnexal ostial nevus.[5] The finding of a keratin plug containing the parakeratotic cornoid lamella overlying the dilated acrosyringeal duct or hair follicle is the most confirmatory diagnostic finding. However, identifying the spatial relationship between a bigger keratin plug within the tiny superficial portion of the eccrine duct is considered a very challenging task. Many sections, in different directions, may be required to identify this. Dilatation or hyperplasia of acrosyringial ducts or presence of a follicle just underlying the cornoid lamella is important clues to the diagnosis. Absence (or hypoplasia) of granular cell layer at the bottom of the plug, and dyskeratotic keratinocytes in the epidermis are also considered helpful features that may assist in the diagnosis.
Role of dermatoscopy was found to be very crucial and reflected its more frequent use as a diagnostic tool. It helped us to assess the actual extent of the lesion beyond the invagination. Numerous densely packed distinct white dots that most possibly represented dilated and hypertrophic appendageal ducts are major histological finding in porokeratotic adnexal ostial nevus that is detected under the dermatoscope. Thus, dermatoscopy can be an important noninvasive alternate tool to elicit these abnormalities in porokeratotic adnexal ostial nevus.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Marsden RA, Fleming K, Dawber RP. Comedo naevus of the palm – a sweat duct naevus? Br J Dermatol 1979;101:717-22.
[Google Scholar]
|
| 2. |
Abell E, Read SI. Porokeratotic eccrine ostial and dermal duct naevus. Br J Dermatol 1980;103:435-41.
[Google Scholar]
|
| 3. |
Goddard DS, Rogers M, Frieden IJ, Krol AL, White CR Jr., Jayaraman AG, et al. Widespread porokeratotic adnexal ostial nevus: clinical features and proposal of a new name unifying porokeratotic eccrine ostial and dermal duct nevus and porokeratotic eccrine and hair follicle nevus. J Am Acad Dermatol 2009;61:1060.e1-4.
[Google Scholar]
|
| 4. |
Levinsohn JL, McNiff JM, Antaya RJ, Choate KA. A somatic p.G45E GJB2 mutation causing porokeratotic eccrine ostial and dermal duct nevus. JAMA Dermatol 2015;151:638-41.
[Google Scholar]
|
| 5. |
Titeux M, Mendonça V, Décha A, Moreira E, Magina S, Maia A, et al. Keratitis-ichthyosis-deafness syndrome caused by GJB2 maternal mosaicism. J Invest Dermatol 2009;129:776-9.
[Google Scholar]
|
Fulltext Views
4,365
PDF downloads
2,464





