Translate this page into:
High level of gamma-glutamyltransferase is a possible risk factor for psoriasis: A nationwide population-based cohort study
Corresponding author: Dr. Ji Hyun Lee, Department of Dermatology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Banpodaero, Seoul, Republic of Korea, yiji1@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Oh JW, Han KD, Doh JY, Gee HY, Lee JH. High level of gamma-glutamyltransferase is a possible risk factor for psoriasis: A nationwide population-based cohort study. Indian J Dermatol Venereol Leprol. 2025;91:23-30. doi: 10.25259/IJDVL_42_2023
Abstract
Background
Gamma-glutamyl transferase (GGT) has been associated with coronary heart disease, diabetes mellitus, and hypertension, but its association with psoriasis has not yet been elucidated.
Aims
We conducted this study to determine the association between the risk of psoriasis and the serum GGT.
Methods
We conducted a nationwide population-based study. A total of 9,939,350 people met the enrolment criteria. The study population was classified into four groups based on GGT levels and the risk of psoriasis was calculated for each group.
Results
The incidence rates of psoriasis per 1,000 person-years were 2.96105 and 3.68577 in the lowest and highest GGT groups, respectively. After adjusting for age, sex, income, diabetes mellitus, hypertension, dyslipidemia, smoking, alcohol intake, exercise, and body mass index, the highest GGT group showed a significantly increased risk of developing psoriasis (hazard ratio: 1.057, 95% confidence interval: 1.044–1.07). This risk of psoriasis was significantly higher among the old age group (hazard ratio: 1.162, 95% confidence interval: 1.128–1.197) and women (hazard ratio: 1.14, 95% confidence interval: 1.117–1.164).
Limitations
The limitations of this study included the retrospective design, International Classification of Diseases code-based diagnosis, small hazard ratio, and non-availability of data on covariates.
Conclusion
The GGT level was found to be an independent risk factor for developing psoriasis.
Keywords
gamma-glutamyltransferase
GGT
psoriasis
risk factor
cohort study
Introduction
Psoriasis is an immune-mediated, chronic inflammatory skin condition affecting approximately 2% of the world’s population.1 Due to its chronic and recurrent nature, psoriasis can profoundly influence the patient’s physical and mental health, and quality of life.2,3
Recent studies on the complex mechanisms and biologics of psoriasis have resulted in considerable changes in the management of psoriasis.4 The high prevalence of psoriasis in adults (2–4%),5 comorbidities with psoriasis,6 and the associated economic burden7 have important public health implications. Identifying the risk factors for a disease and planning strategies for risk factor modification could provide many benefits in terms of prevention and treatment.8–10 Known risk factors of psoriasis include obesity, tobacco, infections, medications, and family history.11,12 However, the risk factors for psoriasis have not yet been fully elucidated. As a systemic inflammatory disease, psoriasis is associated with cardiovascular diseases (CVD), diabetes mellitus (DM), inflammatory bowel disease, and metabolic syndrome.13 Interestingly, two large prospective cohort studies showed that gamma-glutamyltransferase (GGT) had a graded positive association with coronary heart disease (CHD) in the general population of Europe.14,15 In this study, the high GGT group of patients showed an increased CVD risk (hazard ratio (HR): 1.64, 95% confidence interval (CI): 1.35–2.0) and acute coronary events (HR: 2.34, 95% CI: 1.23–4.44). Prospective epidemiological studies have also reported that serum GGT level could predict CVD.16 Other prospective studies also showed that GGT is an independent risk factor for type 2 DM, hypertension, and chronic kidney disease (CKD).17,18 However, the association between the risk of developing psoriasis and the GGT level has not yet been thoroughly investigated. Therefore, the objective of this study was to determine the association between the GGT level and risk of psoriasis using data from the Korean National Health Insurance Service (KNHIS) database.
Materials and Methods
Data source
Baseline data were obtained from the KNHIS which is managed by the Korean government and includes almost 100% of the South Korean population.19 All South Koreans are eligible to participate in regular health check-ups. The medical information of all South Koreans was stored in the KNHIS. The disease data were classified according to the 10th revision of the International Classification of Diseases (ICD-10). All data in the KNHIS database are reidentified and allotted a unique number to prevent disclosure of personal identifiable information of the patients. This study was approved by the Institutional Review Board of the Catholic University of Korea (approval no. KC21ZISI0078).
Study population
The initial data set included 10,585,852 Korean adults aged 20 years or more based on baseline (2009), who underwent health examinations annually. To accurately evaluate the risk of psoriasis according to changes in the serum GGT level, only patients newly diagnosed with psoriasis were included. A total of 260,399 individuals with a diagnosis of psoriasis at the baseline were excluded and 386,103 individuals who had missing information were excluded from the study.
Psoriasis was diagnosed with records (L40) identified by the ICD-10 codes and these diagnosed cases were derived from the outpatients and inpatients. Finally, 9,939,350 adults were included in the analysis. The mean (±standard deviation) follow-up duration of the study population was 9.06 ± 1.35 years. New-onset psoriasis was observed in 302,035 individuals during the follow-up period [Figure 1].
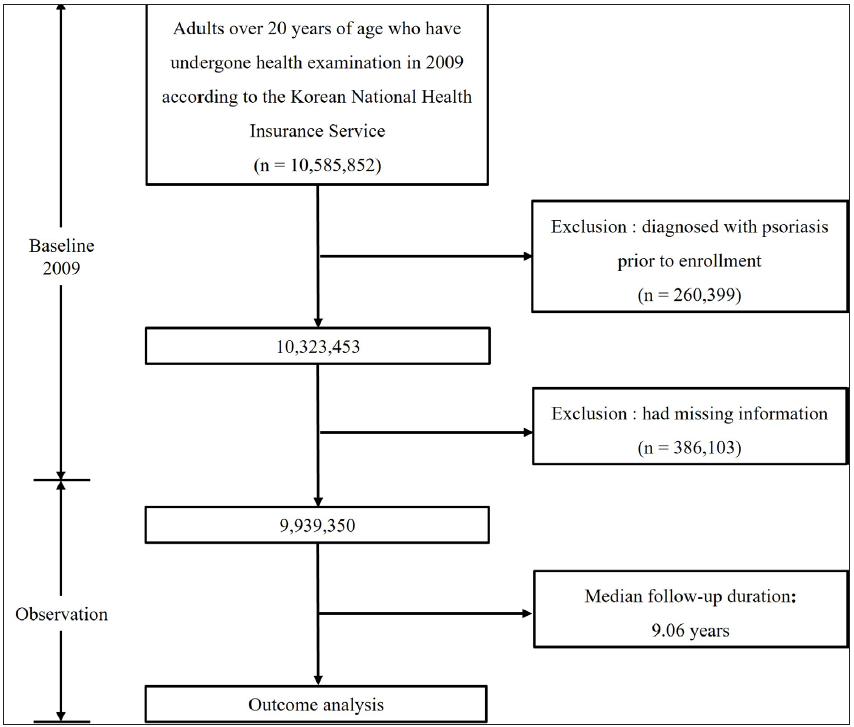
- Flowchart of the enrolment of study population in this study.
Data collection
We included data from 9,939,350 adults who underwent health examinations, including the measurement of serum GGT levels. The main outcome was the occurrence of psoriasis. The KNHIS regular medical health examination programme includes data concerning variables that can alter the general health status of patients including sex, age, DM, hypertension, dyslipidaemia, smoking habits, alcohol intake, and exercise status. Patients were classified according to their smoking habits as non-smokers, ex-smokers, and current smokers. We defined alcohol intake using three categories: none (no alcoholic drinks consumed within the past year), mild (<30g pure alcohol per day), and heavy (≥30g pure alcohol per day). We defined regular exercise as ≥1day per week of moderate or intensive exercise. Venous blood samples for the measurement of fasting glucose, lipid profiles and liver enzyme levels were obtained at each health examination after an overnight fast. Body mass index (BMI) was calculated by dividing the body weight (kg) by the squared value of the height in metres (m2). The health examinations also included measurements of the systolic and diastolic blood pressures and the waist circumference. Comorbid DM, hypertension, and dyslipidemia were defined according to the questionnaire or by both diagnosis codes and associated prescribed medication during the study period. DM was defined as (i) one or more claims/year for antidiabetic prescription under ICD-10 codes E11-14 or (ii) fasting glucose of more than 126 mg/dL. Hypertension was defined as (i) one or more claims/year for antihypertensive prescription under ICD-10 codes I10–I13, I15, or (ii) systolic/diastolic blood pressures of more than 140/90 mmHg. Dyslipidemia was defined as (i) one or more claims/year for an anti-hyperlipidemic prescription under ICD-10 code E78 or (ii) total cholesterol of more than 6.21 mmol/L (240 mg/dL).
Statistical analysis
The study population was classified into four groups according to the GGT level using £25 (Q1, ∼15 IU/L), 25∼50 (Q2, 16∼23 IU/L), 50∼75 (Q3, 24∼39 IU/L), and 75∼100 (Q4, 40 IU/L∼) percentile as cut-off points. In other words, the group with the highest GGT level (Q4) was defined as the highest quartile. Each group was compared by using an analysis of variance [Table 1]. The data are presented as means ± standard deviations. The incidence rate of psoriasis was calculated by dividing the total number of incident cases of psoriasis by the entire follow-up duration (person-years).
| GGT | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| n | 2422766 | 2611671 | 2438419 | 2466494 | |
| Age (years) | 45.06 ± 14.85 | 47.55 ± 14.82 | 48.19 ± 13.96 | 47.42 ± 12.5 | <.0001 |
| 20–39 | 876961 (36.2) | 825723 (31.62) | 723118 (29.66) | 715004 (28.99) | <.0001 |
| 40–64 | 1249630 (51.58) | 1390651 (53.25) | 1366711 (56.05) | 1493242 (60.54) | |
| ≥65 | 296175 (12.22) | 395297 (15.14) | 348590 (14.3) | 258248 (10.47) | |
| Sex, male | 477622 (19.71) | 1209993 (46.33) | 1681580 (68.96) | 2096230 (84.99) | <.0001 |
| Diabetes mellitus | 95214 (3.93) | 175765 (6.73) | 238721 (9.79) | 345555 (14.01) | <.0001 |
| Hypertension | 396849 (16.38) | 625495 (23.95) | 724454 (29.71) | 898543 (36.43) | <.0001 |
| Dyslipidaemia | 243487 (10.05) | 396973 (15.2) | 470614 (19.3) | 607004 (24.61) | <.0001 |
| Smoking | <.0001 | ||||
| Non-smoker | 2061943 (85.11) | 1771111 (67.82) | 1230174 (50.45) | 844493 (34.24) | |
| Ex-smoker | 147485 (6.09) | 317828 (12.17) | 442973 (18.17) | 514710 (20.87) | |
| Current smoker | 213338 (8.81) | 522732 (20.02) | 765272 (31.38) | 1107291 (44.89) | |
| Alchol intake (drink) | <.0001 | ||||
| Non | 1709307 (70.55) | 1565906 (59.96) | 1142162 (46.84) | 698900 (28.34) | |
| Mild | 684250 (28.24) | 959515 (36.74) | 1111355 (45.58) | 1276772 (51.76) | |
| Heavy | 29209 (1.21) | 86250 (3.3) | 184902 (7.58) | 490822 (19.9) | |
| Regular exercise | 398816 (16.46) | 480622 (18.4) | 467620 (19.18) | 452726 (18.36) | <.0001 |
| BMI (kg/m2) | 22.29 ± 2.84 | 23.21 ± 3.04 | 24.21 ± 3.46 | 25.13 ± 3.72 | <.0001 |
| Waist circumference (cm) | 74.61 ± 8.45 | 78.55 ± 8.61 | 82.3 ± 8.57 | 85.48 ± 8.51 | <.0001 |
| Total Cholesterol (mg/dL) | 185.73 ± 38.18 | 192.49 ± 40.45 | 198.1 ± 41.09 | 204.59 ± 43.61 | <.0001 |
| Glucose (mg/dL) | 91.8 ± 16.24 | 94.93 ± 20.44 | 98.44 ± 24.72 | 103.88 ± 30.25 | <.0001 |
| SBP (mmHg) | 117.26 ± 14.49 | 121.16 ± 14.66 | 124.04 ± 14.44 | 127.34 ± 14.75 | <.0001 |
| DBP (mmHg) | 72.84 ± 9.54 | 75.28 ± 9.63 | 77.32 ± 9.64 | 79.87 ± 10.05 | <.0001 |
| TG (mg/dL) | 94.39 ± 58.65 | 115.05 ± 70.73 | 141.08 ± 86.62 | 187.67 ± 120.84 | <.0001 |
| HDL (mg/dL) | 59.43 ± 33.27 | 57.32 ± 34.36 | 54.8 ± 32.13 | 54.37 ± 32 | <.0001 |
| LDL (mg/dL) | 126.67 ± 349.4 | 120.09 ± 178.87 | 120.98 ± 139.11 | 117.33 ± 120.65 | <.0001 |
| GFR (mL/min/1.73 m2) | 83.14 ± 26.47 | 83.72 ± 26.84 | 87.78 ± 28.32 | 93.78 ± 30.1 | <.0001 |
| AST (IU/L) | 20.44 ± 7.41 | 22.36 ± 8.69 | 24.95 ± 10.35 | 34.19 ± 27.34 | <.0001 |
| ALT (IU/L) | 15.72 ± 7.29 | 19.65 ± 9.81 | 25.66 ± 9.81 | 40.91 ± 35.11 | <.0001 |
Continuous variables are presented as mean ± SD and categorical variables as number (percentage).
The study population was classified into quartile groups according to GGT level: Q1 (≤15 IU/L), Q2 (16–23 IU/L), Q3 (24–39 IU/L), and Q4 (≥40 IU/L)
GGT, Gamma-glutamyltransferase; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; GFR, glomerular filtration rate; AST, aspartate transaminase; ALT, alanine transaminase; n: number of patients.
We performed multivariable Cox proportional hazard regression analyses to evaluate the associations of GGT level changes with the risk of psoriasis. All the covariates incorporated into the Cox model were found to adhere to the proportional hazards assumption. The multivariable model included age, sex, income, DM, hypertension, dyslipidemia, smoking status, alcohol consumption, physical activity, and BMI. Patients were subdivided into three groups based on age (20–39, 40–64, and ≥65 years). In addition, we performed stratified analyses by age, sex, DM, hypertension, dyslipidemia, smoking habit, and BMI. All statistical analyses were performed using Statistical Analysis System (SAS) software (version 9.4, SAS Institute, Cary, NC, USA). P values < 0.05 were considered statistically significant.
Results
Characteristics of the study population according to the GGT level
We identified 9,939,350 people who met the enrolment criteria. We divided these people into four subgroups according to their GGT levels. The number of people enrolled in each group did not differ significantly. The proportion of men was high in the highest GGT group (84.99%). In the GGT groups, GGT showed a correlation with current smoking habits, mild and heavy alcohol intake, BMI, waist circumference, total cholesterol, glucose, systolic blood pressure, diastolic blood pressure, triglyceride, and aspartate transaminase and alanine transaminase levels (p < 0.001). The proportion of individuals aged 65 years and older was lower in the highest GGT group (10.47%) than in the lowest GGT group (12.22%) [Table 1].
Incidence rate and risk of psoriasis among the study population according to GGT levels
The incidence rate of psoriasis was 2.96105 cases/1,000 person-years in the lowest GGT group and 3.68577 cases/1,000 person-years in the highest GGT group. The highest GGT group showed a significantly increased risk of developing psoriasis [HR: 1.245, 95% CI: 1.233–1.258] compared to the lowest GGT group [Table 2, Figure 2]. In each GGT group, the incidence rate of psoriasis was 2.96105, 3.24483, 3.52733 and 3.68577 (cases/1,000 person-years). The incidence rate of psoriasis was higher in the group with a high GGT group compared to the Q1 group. After adjusting for age, sex, income, DM, hypertension, dyslipidaemia, smoking, alcohol intake, exercise status, and BMI, individuals in the highest GGT group were at a 5.7% increased risk for psoriasis (HR: 1.057, 95% CI: 1.044–1.07) compared to the individuals in the Q1 group and this dose–response association also remained valid.
| GGT | N | Psoriasis(n) | Person-Years | IR (per 1,000) | MODEL 1 | MODEL 2 | MODEL 3 |
|---|---|---|---|---|---|---|---|
| Q1 | 2,422,766 | 65,508 | 22,123,243.98 | 2.96105 | 1 (ref.) | 1 (ref.) | 1 (ref.) |
| Q2 | 2,611,671 | 77,053 | 23,746,364.2 | 3.24483 | 1.096 (1.085, 1.107) | 1.007 (0.996, 1.018) | 1.006 (0.995, 1.017) |
| Q3 | 2,438,419 | 77,877 | 22,078,191.33 | 3.52733 | 1.191 (1.179, 1.204) | 1.04 (1.028, 1.052) | 1.038 (1.026, 1.049) |
| Q4 | 2,466,494 | 81,597 | 22,138,356.36 | 3.68577 | 1.245 (1.233, 1.258) | 1.06 (1.047, 1.073) | 1.057 (1.044, 1.07) |
The study population was classified into quartile groups according to GGT level: Q1 (≤15 IU/L), Q2 (16–23 IU/L), Q3 (24–39 IU/L), and Q4 (≥40 IU/L).
MODEL 1: Unadjusted
MODEL 2: Age, Sex, Income, Diabetes mellitus, Hypertension, Dyslipidaemia, Smoke, Drink, Exercise
MODEL 3: Age, Sex, Income, Diabetes mellitus, Hypertension, Dyslipidaemia, Smoke, Drink, Exercise, BMI
BMI, body mass index; GGT, Gamma-glutamyltransferase; IR, Incidence rate; N, number of people.
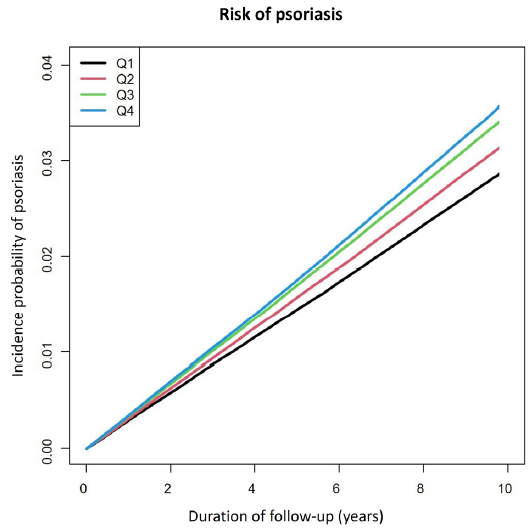
- Kaplan–Meier plots stratified by Gamma-glutamyltransferase (GGT) categories. The x-axis represents the duration of follow-up, while the y-axis illustrates the incidence probability of psoriasis. The study population was classified into quartile groups according to GGT level: Q1 (≤15 IU/L), Q2 (16 -23 IU/L), Q3 (24 -39 IU/L), and Q4 (≥40 IU/L).
Next, we performed additional stratified analyses to find specific risk groups [Table 3]. The association between the serum GGT level and psoriasis was observed in women in the older age groups (40–64 and ≥65 years), those with DM, hypertension, and/or dyslipidemia, and were current smokers. In the older age groups, the high GGT level group showed an increased risk for psoriasis (HR: 1.162, 95% CI: 1.128–1.197, P for interaction <0.001) compared to the individuals in the reference group. Also, in the women’s group, high GGT levels showed increased risk for psoriasis (HR: 1.14, 95% CI: 1.117–1.164, P for interaction: 0.0028) compared to the reference group.
| GGT | N | Psoriasis (n) | Person-Years | IR (per 1,000) | Model 3 | P for interaction | ||
|---|---|---|---|---|---|---|---|---|
| Age | 20∼39 | Q1 | 876,961 | 21,561 | 8,072,569.09 | 2.6709 | 1 (ref.) | <.0001 |
| Q2 | 825,723 | 20,123 | 7,602,487.08 | 2.6469 | 1.013 (0.993, 1.034) | |||
| Q3 | 723,118 | 17,972 | 6,649,086.88 | 2.70293 | 1.028 (1.004, 1.052) | |||
| Q4 | 715,004 | 18,289 | 6,552,785.25 | 2.79103 | 1.027 (1.001, 1.054) | |||
| 40∼64 | Q1 | 1,249,630 | 34,207 | 11,498,415.02 | 2.97493 | 1 (ref.) | ||
| Q2 | 1,390,651 | 42,126 | 12,747,580.56 | 3.30463 | 1.03 (1.015, 1.045) | |||
| Q3 | 1,366,711 | 44,887 | 12,472,190.00 | 3.59897 | 1.062 (1.046, 1.079) | |||
| Q4 | 1,493,242 | 51,439 | 13,479,566.48 | 3.81607 | 1.095 (1.077, 1.113) | |||
| 65∼ | Q1 | 296,175 | 9,740 | 2,552,259.86 | 3.81623 | 1 (ref.) | ||
| Q2 | 395,297 | 14,804 | 3,396,296.56 | 4.35887 | 1.044 (1.018, 1.072) | |||
| Q3 | 348,590 | 15,018 | 2,956,914.46 | 5.07894 | 1.115 (1.085, 1.145) | |||
| Q4 | 258,248 | 11,869 | 2,106,004.63 | 5.63579 | 1.162 (1.128, 1.197) | |||
| Sex | Male | Q1 | 477,622 | 13,979 | 4,313,086.99 | 3.24107 | 1 (ref.) | 0.0028 |
| Q2 | 1,209,993 | 36,863 | 10,952,495.67 | 3.36572 | 1.021 (1.001, 1.041) | |||
| Q3 | 1,681,580 | 54,477 | 15,196,673.72 | 3.5848 | 1.05 (1.03, 1.07) | |||
| Q4 | 2,096,230 | 69,429 | 18,804,283.83 | 3.69219 | 1.065 (1.045, 1.085) | |||
| Female | Q1 | 1,945,144 | 51,529 | 17,810,156.99 | 2.89324 | 1 (ref.) | ||
| Q2 | 1,401,678 | 40,190 | 12,793,868.53 | 3.14135 | 1.041 (1.027, 1.055) | |||
| Q3 | 756,839 | 23,400 | 6,881,517.61 | 3.40041 | 1.086 (1.069, 1.104) | |||
| Q4 | 370,264 | 12,168 | 3,334,072.53 | 3.64959 | 1.14 (1.117, 1.164) | |||
| DM | No | Q1 | 2,261,183 | 59,834 | 20,702,881.54 | 2.89013 | 1 (ref.) | <.0001 |
| Q2 | 2,344,672 | 67,069 | 21,398,431.93 | 3.1343 | 1.014 (1.002, 1.025) | |||
| Q3 | 2,107,160 | 64,405 | 19,168,208.72 | 3.35999 | 1.043 (1.031, 1.056) | |||
| Q4 | 2,026,836 | 64,190 | 18,311,476.86 | 3.50545 | 1.068 (1.054, 1.083) | |||
| Yes | Q1 | 161,583 | 5,674 | 1,420,362.44 | 3.99476 | 1 (ref.) | ||
| Q2 | 266,999 | 9,984 | 2,347,932.27 | 4.25225 | 1.002 (0.969, 1.035) | |||
| Q3 | 331,259 | 13,472 | 2,909,982.61 | 4.62958 | 1.055 (1.022, 1.09) | |||
| Q4 | 439,658 | 17,407 | 3,826,879.50 | 4.54861 | 1.049 (1.014, 1.085) | |||
| Hypertension | No | Q1 | 2,019,487 | 53,036 | 18,531,931.93 | 2.86187 | 1 (ref.) | <.0001 |
| Q2 | 1,976,125 | 55,560 | 18,083,380.60 | 3.07243 | 1.018 (1.006, 1.031) | |||
| Q3 | 1,704,330 | 50,813 | 15,551,702.79 | 3.26736 | 1.044 (1.03, 1.058) | |||
| Q4 | 1,559,307 | 48,200 | 14,125,002.92 | 3.41239 | 1.065 (1.049, 1.081) | |||
| Yes | Q1 | 403,279 | 12,472 | 3,591,312.04 | 3.47283 | 1 (ref.) | ||
| Q2 | 635,546 | 21,493 | 5,662,983.60 | 3.79535 | 1.035 (1.012, 1.058) | |||
| Q3 | 734,089 | 27,064 | 6,526,488.54 | 4.14679 | 1.088 (1.064, 1.112) | |||
| Q4 | 907,187 | 33,397 | 8,013,353.44 | 4.16767 | 1.098 (1.073, 1.124) | |||
| Dyslipidaemia | No | Q1 | 2,140,906 | 56,664 | 19,572,722.89 | 2.89505 | 1 (ref.) | <.0001 |
| Q2 | 2,162,427 | 61,683 | 19,692,799.90 | 3.13226 | 1.014 (1.002, 1.026) | |||
| Q3 | 1,911,375 | 58,351 | 17,341,667.60 | 3.36479 | 1.044 (1.031, 1.057) | |||
| Q4 | 1,791,728 | 56,668 | 16,094,532.83 | 3.52095 | 1.066 (1.051, 1.08) | |||
| Yes | Q1 | 281,860 | 8,844 | 2,550,521.09 | 3.46753 | 1 (ref.) | ||
| Q2 | 449,244 | 15,370 | 4,053,564.30 | 3.79172 | 1.022 (0.996, 1.05) | |||
| Q3 | 527,044 | 19,526 | 4,736,523.73 | 4.12243 | 1.063 (1.035, 1.091) | |||
| Q4 | 674,766 | 24,929 | 6,043,823.53 | 4.12471 | 1.064 (1.035, 1.093) | |||
| Current smoker | No | Q1 | 2,209,428 | 59,201 | 20,189,247.03 | 2.9323 | 1 (ref.) | <.0001 |
| Q2 | 2,088,939 | 60,964 | 19,008,412.70 | 3.20721 | 1.004 (0.993, 1.016) | |||
| Q3 | 1,673,147 | 53,065 | 15,153,031.05 | 3.50194 | 1.033 (1.02, 1.046) | |||
| Q4 | 1,359,203 | 45,385 | 12,196,796.62 | 3.72106 | 1.063 (1.048, 1.078) | |||
| Yes | Q1 | 213,338 | 6,307 | 1,933,996.95 | 3.26112 | 1 (ref.) | ||
| Q2 | 522,732 | 16,089 | 473,7951.50 | 3.39577 | 1.04 (1.01, 1.071) | |||
| Q3 | 765,272 | 24,812 | 6,925,160.28 | 3.58288 | 1.074 (1.044, 1.105) | |||
| Q4 | 1,107,291 | 36,212 | 9,941,559.74 | 3.64249 | 1.08 (1.049, 1.111) | |||
| BMI | < 25 | Q1 | 2,025,006 | 53,745 | 18,490,083.97 | 2.90669 | 1 (ref.) | 0.0007 |
| Q2 | 1,931,451 | 55,451 | 17,554,087.97 | 3.15887 | 1.005 (0.993, 1.018) | |||
| Q3 | 1,512,014 | 46,798 | 13,665,208.70 | 3.42461 | 1.031 (1.017, 1.045) | |||
| Q4 | 1,224,774 | 40,092 | 10,906,155.86 | 3.67609 | 1.059 (1.043, 1.075) | |||
| ≥ 25 | Q1 | 397,760 | 11,763 | 3,633,160.01 | 3.23768 | 1 (ref.) | ||
| Q2 | 680,220 | 21,602 | 6,192,276.23 | 3.48854 | 1.019 (0.996, 1.043) | |||
| Q3 | 926,405 | 31,079 | 8,412,982.63 | 3.69417 | 1.05 (1.026, 1.074) | |||
| Q4 | 1,241,720 | 41,505 | 11,232,200.50 | 3.69518 | 1.05 (1.026, 1.075) |
MODEL 3: Age, Sex, Income, DM, Hypertension, Dyslipidaemia, Smoke, Drink, Exercise, BMI; N, number of patients, SD, Standard deviation
The study population was classified into quartile groups according to GGT level: Q1 (≤15 IU/L), Q2 (16 -23 IU/L), Q3 (24 -39 IU/L), and Q4 (≥40 IU/L).
GGT, Gamma-glutamyltransferase; BMI, body mass index; DM, diabetes mellitus; IR, incidence rate; N, number of people.
Discussion
In this study, our results showed a higher incidence rate of psoriasis in the high serum GGT group. The serum GGT level had a dose-dependent association with the risk of developing psoriasis. Even after adjusting for covariates, GGT level was found to be an independent risk factor for the development of psoriasis. This positive association was observed in all age groups and both sexes but was especially pronounced in the older age group and in women. While the link between psoriasis and GGT levels has not yet been well studied, two large prospective cohort studies from Europe showed a positive association between GGT levels and CHD.14,15 Also, many prospective studies have reported that the GGT level predicts various diseases: CHD, type 2 DM, hypertension and CKD. In a prospective cohort study, there was a strong association between the GGT level and CHDs even in non-drinkers.16 In Lee’s study, a strong relationship between the GGT level and risk of DM and hypertension was observed, even in non-drinkers and individuals without abnormal liver enzymes.18 Although the GGT level has been used widely as an index for alcohol intake or liver abnormality, neither alcohol nor hepatic dysfunction explained the observed association between the GGT level and DM. In our study too, adjustment for alcohol consumption did not change the relationship between the GGT level and the risk of psoriasis. The association of GGT level with these diseases, which are known comorbidities of psoriasis, and our results based on big data suggest that the association between the risk of psoriasis and the GGT level is quite reliable.
The role of glutamyl transferase as an oxidative stress marker could possibly explain the role of glutamyl transferase as an independent risk factor for psoriasis. Glutamyl transferase plays an important role in the metabolism of glutathione which is the most abundant intracellular anti-oxidant.20 GGT metabolises extracellular glutathione to be reutilised for intracellular glutathione re-synthesis.21 In an animal study, it was confirmed that the GGT level increased when glutathione was insufficient and the GGT level was restored when glutathione was supplemented.22 In GGT gene inactivated cases, intracellular levels of glutathione in animals are 50% of the levels observed in animals with the wild-type gene, whereas plasma and urine glutathione levels are markedly elevated in both humans and mice.23–25 Another research showed that oxidative stress was increased in the kidneys of GGT-deficient mice.25 The findings in both humans and mice are consistent with the major role played by GGT in glutathione metabolism.24,25
Although the major underlying pathogenesis of psoriasis is thought to be by the immune cell-derived interleukin(IL)-23/IL-17 cytokine axis, in light of its chronic and inflammatory nature, there is accumulating evidence linking oxidative stress to the pathogenesis of psoriasis.26 In the imiquimod-induced psoriasis mouse model, oxidative stress was increased and the anti-oxidative system was found to be aberrant.27 Another set of research data also showed an insufficient anti-oxidant system in the serum and tissue of patients suffering from psoriasis.28 Supplementation with anti-oxidants resulted in significant improvement in patients with severe erythrodermic and arthropathic forms of psoriasis.29 Tumour necrosis factor alpha which is an important factor in the pathogenesis of psoriasis leads to the formation of reactive oxygen species in primary human keratinocytes.30 Based on these data, it can be concluded that there is an aberrant anti-oxidant system in psoriasis and psoriasis is an oxidative stress condition. Further studies on factors that influence the GGT level might be helpful to elucidate the mechanism by which GGT affects psoriasis.
Our results showed a stronger association between the serum GGT level and psoriasis in older patients and women. However, it can be challenging to explain these tendencies. In South Korea, the incidence of psoriasis in old age was much lower than that in young age (Odds ratio: 0.80, 95% CI: 0.78–0.83, p < 0.0001) and the men to women ratio were 1.3:1.31 Nevertheless, the actual incidence rate was higher and a stronger tendency was shown in old age. In women, the incidence rate was lower than in men in the lowest GGT group (2.89324 cases/1,000 person-years vs 3.24107 cases/1,000 person-years), but similar to men in the highest GGT group (3.64959 cases/1,000 person-years vs 3.69219 cases/1,000 person-years). Elderly Korean women reported much lower amounts of alcohol consumption than young Korean men.32,33 Although there is a possibility that alcohol intake could influence GGT, old age and women showed a stronger tendency after adjusting for these confounding factors. This suggests that it may be used as a more sensitive indicator in this group. Our study has certain strengths. Our study cohort was representative of the entire Korean population; therefore, the findings are generalised to the public in Korea. In addition, we conducted a stratification analysis to check for a more specific risk group. There are also several limitations to this study. First, there is a possibility of misclassification of disease in the ICD-10 code-based datasets in KNHIS. Second, our dataset lacked information on genetic factors, drug history, other medical history, and time-varying confounders which could impact the GGT status or psoriasis risk. Nevertheless, we used a multivariable Cox proportional hazards model and adjusted for all possible covariates such as smoking, alcohol intake, and BMI. Third, because our study was not prospective, the exact causal relationship was not accurately estimated. To avoid the possible effects of reverse causality, we established a 1-year washout period and excluded persons with pre-existing psoriasis. Fourth, the highest GGT group had a statistically significant increased HR for developing psoriasis, but it was not high.
Conclusion
We observed a significant increase in the incidence of psoriasis in individuals with a high serum GGT level. The association persisted even after adjusting for covariates. This relation was pronounced among older women. These results suggest that the role of GGT as an oxidative stress marker possibly makes it a marker for psoriasis risk. Since oxidative stress plays an important role in the pathogenesis of psoriasis, the measurement of serum GGT in older patients and women may be helpful to predict the future risk of psoriasis.
Ethical approval
The Institutional Review Board of the Catholic University of Korea (approval no. KC21ZISI0078) approved the study protocol.
Declaration of patient consent
Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Financial support and sponsorship
National Research Foundation of Korea (NRF) grant funded by the South Korean government (No. NRF-2018R1A5A2025079).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401-7.
- [CrossRef] [PubMed] [Google Scholar]
- Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213-26.
- [CrossRef] [PubMed] [Google Scholar]
- Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-85.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of psychiatric diseases among patients with psoriasis in Korea: A 12‐year nationwide population‐based cohort study. J Dermatol. 2021;48:1763-71.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of hospital readmission in United States adults with psoriasis. J Am Acad Dermatol. 2020;82:902-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of weight loss on the severity of psoriasis: A randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- [CrossRef] [PubMed] [Google Scholar]
- Methotrexate in psoriasis: A systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J Eur Acad Dermatol Venereol. 2011;25:12-8.
- [CrossRef] [PubMed] [Google Scholar]
- Modifiable risk factors and the development of psoriatic arthritis in people with psoriasis. Br J Dermatol. 2020;182:714-20.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945-60.
- [CrossRef] [PubMed] [Google Scholar]
- Smoking and risk of psoriasis: A nationwide cohort study. J Am Acad Dermatol. 2017;77:573-5.
- [CrossRef] [PubMed] [Google Scholar]
- Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-96.
- [CrossRef] [PubMed] [Google Scholar]
- g-Glutamyltransferase as a risk factor for cardiovascular disease mortality: An epidemiological investigation in a cohort of 163,944 Austratian adults. Circ Circulation. 2005;112:2130-7.
- [CrossRef] [PubMed] [Google Scholar]
- Serum g-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atheroscler Atherosclerosis. 2006;189:297-302.
- [CrossRef] [PubMed] [Google Scholar]
- Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28 838 middle-aged men and women. Eur Heart J. 2006;27:2170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association of serum carotenoids and tocopherols with g-glutamyltransferase: The cardiovascular risk development in young adults (CARDIA) study. Clin Chem. 2004;50:582-8.
- [CrossRef] [PubMed] [Google Scholar]
- g-glutamyltransferase is a predictor of incident diabetes and hypertension: The coronary artery risk development in young adults (CARDIA) study. Clin Chem. 2003;49:1358-66.
- [CrossRef] [PubMed] [Google Scholar]
- Trends in diabetes incidence in the last decade based on Korean national health insurance claims data. Endocrinol Metab. 2016;31:292-9.
- [CrossRef] [PubMed] [Google Scholar]
- Serum g-glutamyltransferase: New insights about an old enzyme. J Epidemiol Commuinty Health. 2009;63:884-6.
- [CrossRef] [PubMed] [Google Scholar]
- Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263-355.
- [CrossRef] [PubMed] [Google Scholar]
- DL-methionine supplementation of rice-and-bean diets affects gamma-glutamyltranspeptidase activity and glutathione content in livers of growing rats. Braz J Med Biol Res. 1999;32:483-8.
- [CrossRef] [PubMed] [Google Scholar]
- Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci. 1996;93:7923-6.
- [CrossRef] [PubMed] [Google Scholar]
- Serum gamma-glutamyl transpeptidase deficiency. The Lancet. 1971;297:234-5.
- [CrossRef] [PubMed] [Google Scholar]
- g-Glutamyl transferase (GGT) deficiency in the GGT enu1 mouse results from a single point mutation that leads to a stop codon in the first coding exon of GGT mRNA. Mutagenesis. 1999;14:31-6.
- [CrossRef] [PubMed] [Google Scholar]
- Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:6206.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res. 2012;304:699-706.
- [CrossRef] [PubMed] [Google Scholar]
- The role of oxidants and antioxidants in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:34-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and biochemical effects of coenzyme Q10, vitamin E, and selenium supplementation to psoriasis patients. Nutrition. 2009;25:295-302.
- [CrossRef] [PubMed] [Google Scholar]
- Reactive oxygen species in tumor necrosis factor-a-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of psoriasis in Korea: a population-based epidemiological study using the Korean national health insurance database. Ann Dermatol. 2017;29:761-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trends in alcohol consumption for Korean adults from 1998 to 2018: Korea national health and nutritional examination survey. Nutrients. 2021;13:609.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Alcohol-drinking patterns and metabolic syndrome risk: The 2007 Korean national health and nutrition examination survey. Alcohol. 2011;45:499-505.
- [CrossRef] [PubMed] [Google Scholar]







