Translate this page into:
Imatinib induced melasma-like pigmentation: Report of five cases and review of literature
Correspondence Address:
Rashmi Sarkar
Department of Dermatology, Venereology and Lepreology, Maulana Azad Medical College and Associated Lok Nayak Hospital, New Delhi
India
| How to cite this article: Ghunawat S, Sarkar R, Garg VK. Imatinib induced melasma-like pigmentation: Report of five cases and review of literature. Indian J Dermatol Venereol Leprol 2016;82:409-412 |
Abstract
Imatinib mesylate is a cytotoxic agent that targets tyrosine kinase. Common side effects of this drug include nausea, edema and maculopapular rash. Hypopigmentation is a commonly reported side effect of this drug while hyperpigmentation has rarely been described. We describe five cases of melasma-like pigmentation induced by this anti-cancer drug. Four of the patients were diagnosed with gastrointestinal stromal tumor while one had chronic myeloid leukemia. Patients received imatinib mesylate in a dose of 400 mg daily. Over an average period of 3 months, well defined hyperpigmented macules appeared over the convexities of the face. One of the patients also developed similar pigmentation on the forearm. Other causes of hyperpigmentation were excluded in each patient.Introduction
Imatinib is a tyrosine kinase inhibitor that targets break cluster region-Abelson (BCR-ABL) tyrosine kinase, platelet derived growth factor, and v kit Hardy Zukerman 4 feline sarcoma viral gene homologe (KIT). It is FDA approved for Philadelphia chromosome positive adult chronic myelogenous leukaemia, KIT positive metastatic gastrointestinal stromal tumor (GIST), acute myelogenous leukaemia, myelodysplastic/myeloproliferative diseases, hypereosinoplilic syndrome, aggressive mastocytosis and refractory dermatofibrosarcoma protuberans.[1] Recently, it has also been used in the treatment of metastatic malignant melanoma.[2] Reversible hypopigmentation is a well recognized side effect of this drug, however, hyperpigmentation has been rarely reported. Herein, we report five cases of melasma-like pigmentation caused by this anti-tumor agent. We were able to find two previous reports of similar cases of hyperpigmentation.[1],[3]
Case Reports
Case 1
A 38-year-old man presented with pain in the abdomen for a year. On investigation, he was found to have a gastrointestinal stromal tumor of the duodenum which was confirmed on histopathology. He underwent pancreato-duodenectomy and was started on imatinib, 400mg daily along with ranitidine. One month later, the patient noticed asymptomatic brownish discoloration of his forehead and cheeks [Figure - 1] which was non-progressive. Apart from this, the muco-cutaneous examination was within normal limits. A skin biopsy from the lesion revealed increased basal layer pigmentation along with elastotic degeneration in the upper dermis [Figure - 2].
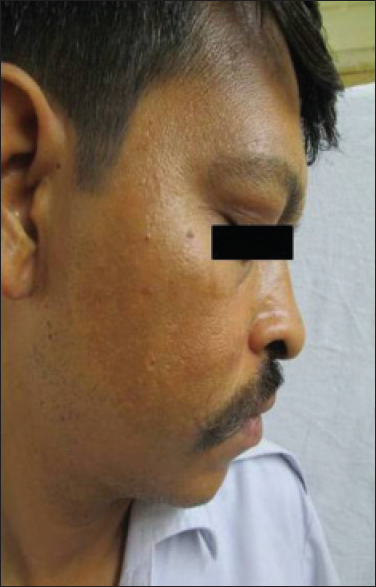 |
| Figure 1: Hyperpigmentation present diffusely over cheek |
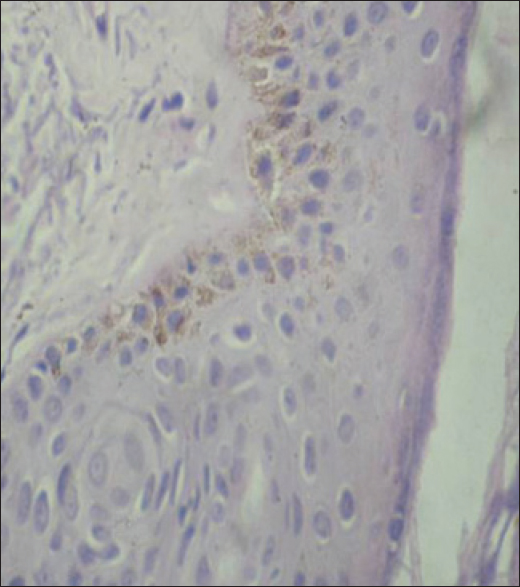 |
| Figure 2: Biopsy from the cheek showing increased basal layer pigmentation (H and E; ×400) |
Case 2
A 40-year-old man presented with complaints of abdominal pain and passage of black tarry stools for 3 years. He was diagnosed with a poorly differentiated gastrointestinal stromal tumor of the jejunum and was started on imatinib 400 mg daily after undergoing jejunectomy. Three months after the start of therapy, the patient noticed the development of well-defined, irregularly shaped, hyperpigmented macules on his forehead and cheeks [Figure - 3] which gradually spread to involve his nose.
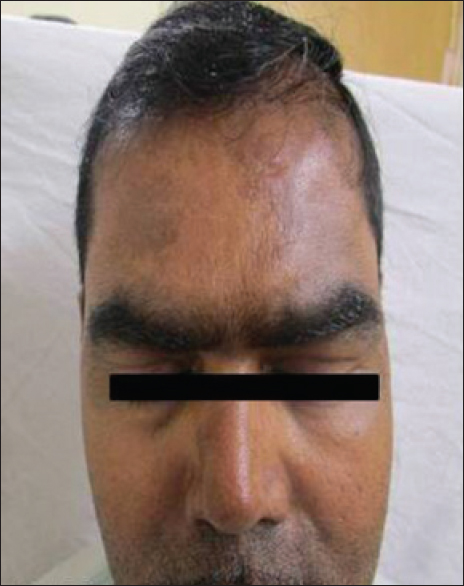 |
| Figure 3: Hyperpigmented macules present over forehead |
Case 3
A 60-year-old man patient presented with intermittent constipation and dull aching abdominal pain for 3 years. On histopathology, he was diagnosed to have a high grade gastrointestinal stromal tumor of the ileocecal junction. The patient underwent bowel resection surgery and was started on imatinib, 400 mg daily along with pantoprazole. There was no history of any other concomitant systemic illness or medication intake. Three months after the start of therapy, he noticed the appearance of dark brown, coalescent, irregular macules forming a reticulate pattern over the convexities of his cheeks [Figure - 4].
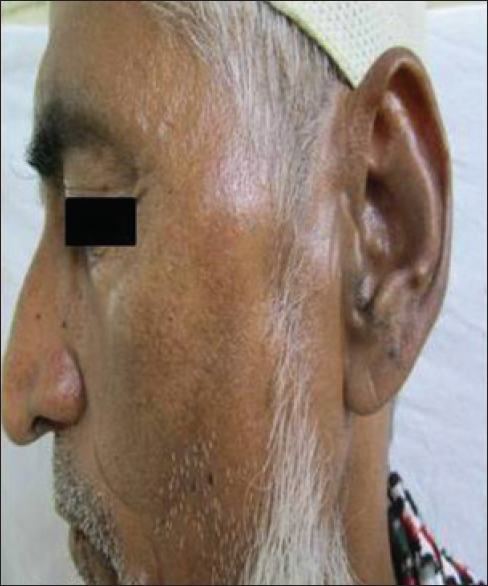 |
| Figure 4: Pigmentation present over cheeks |
Case 4
A 45-year-old woman presented with complaints of easy fatiguability for a year. On investigation, she was found to have chronic myeloid leukemia and was started on imatinib 400 mg daily. After 6 months of therapy, she started noticing brownish pigmentation over her forehead, nose and convexities of both cheeks [Figure - 5]. The pigmentation was gradually progressive and spread to involve the lower face.
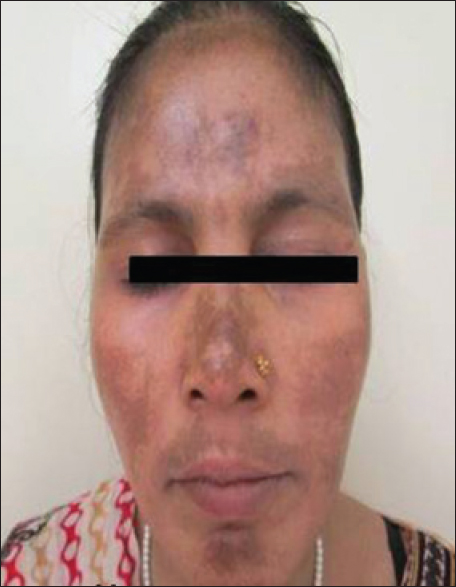 |
| Figure 5: Irregular well defined hyperpigmented macules over facial convexities |
Case 5
A 50-year-old woman presented with abdominal pain and easy fatigability for two years. She was diagnosed to have a jejunal gastrointestinal stromal tumor (high grade) and was started on imatinib, 400 mg daily along with pantoprazole. Six months after the start of treatment, the patient noticed the appearance of irregular, brownish macules with clearly defined borders over her forehead and cheeks. The onset was insidious and the lesions gradually spread to the temples and chin, covering the entire face [Figure - 6]. Subsequently, she noticed similar pigmentation involving both her forearms [Figure - 7].
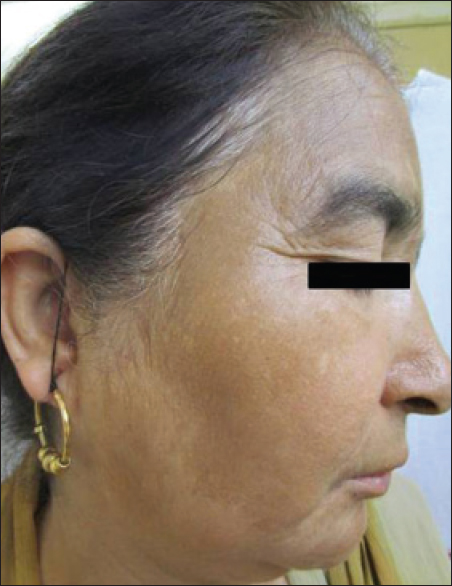 |
| Figure 6: Diffuse brownish pigmentation over cheek |
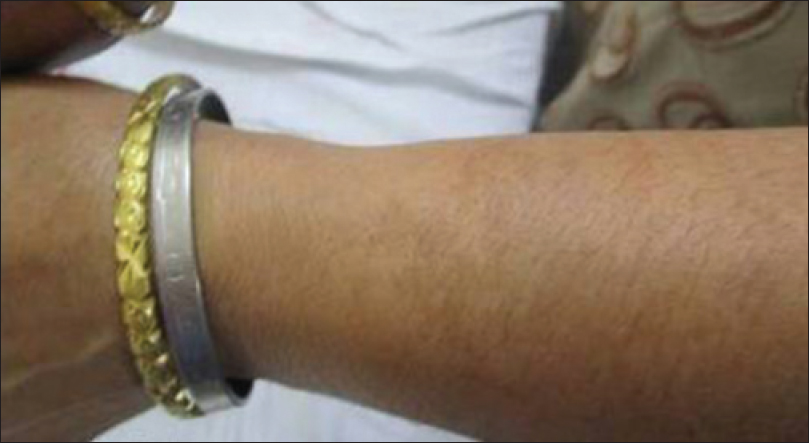 |
| Figure 7: Pigmentation over forearm |
Discussion
Imatinib belongs to a new class of anti-cancer drugs that target the tyrosine kinase receptor. Its systemic side effects are less severe than those seen with other cytotoxic drugs. The most common toxicities are nausea, myalgia, skin rashes and edema. Among the dermatological side effects, maculopapular rash is the most common. Other side effects include xerosis, photosensitivity, angular cheilitis, psoriasiform rash and pigmentary changes. Rarer side effects include acute generalized exanthematous pustulosis, urticaria, lichenoid reaction and painful oral erosions.[4] Among the pigmentary changes seen with this drug, hypopigmentation has been most commonly reported. The drug inhibits melanogenesis via inhibition of the binding of ligands to c-kit receptors. In one study, 41% of patients receiving imatinib were reported to develop hypopigmentation.[5] The morphological variants included generalized skin lightening, vitiligo-like lesions and hair graying.
On the contrary, there are only a few case reports of imatinib-induced hyperpigmentation in the available literature [Table - 1]. Pigmentation associated with imatinib has been described not only in the skin but also involving the palatal mucosa, nails, teeth, hair, and gums.[4],[8],[9],[10],[11] In one series, 3.6% of patients were found to have imatinib-induced pigmentation.[5] So far, the mechanism of this pigmentation remains elusive. It has been attributed to the formation of a drug–melanin metabolite. Other proposed theories include drug-induced cytotoxic response to epidermal 'neo antigen' and the presence of a specific KIT mutation and its interaction with other receptors.[4],[12]
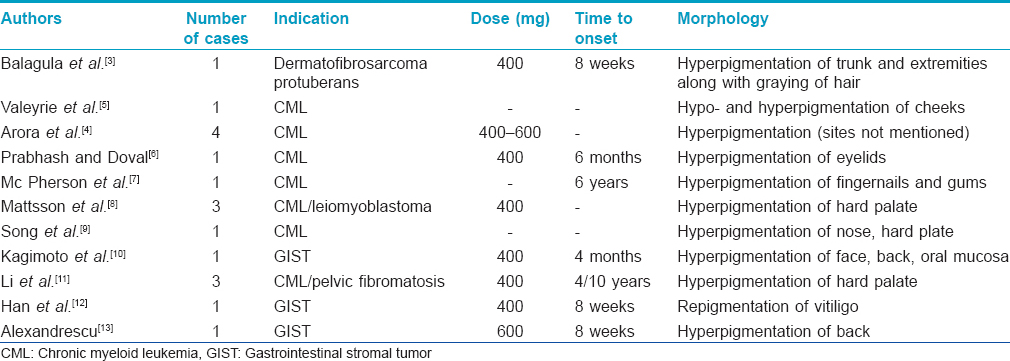
Our patients developed melasma-like pigmentation present predominantly on the forehead and convexities of the cheeks at a mean interval of 3 months after starting therapy. All other causes of pigmentation were ruled out. Of note, Case 3 also developed extrafacial melasma on the forearms. Drugs that have been reported to cause melasma-like pigmentation include oral contraceptive pills, hormone replacement therapy, drugs causing phototoxic and photo-allergic reactions and anti-epilepsy medications.[14],[15] No morphological differences were noted between melasma-like pigmentation due to imatinib, and melasma. The patients were advised about sun protection and were counseled regarding the reversible nature of the pigmentation. One of the patients (case 1) was started on modified Kligman's regimen (0.5% tretinoin + 4% hydroquinone + 0.1% fluocinolone acetonide) along with broad spectrum sunscreen due to his cosmetic worry. An improvement of 30% in Melasma Area and Severity Index (MASI) was noted after 6 weeks of therapy. The patient was lost to follow up after that. Imatinib was not stopped in any patient.
Both hyper- and hypopigmentation have been reported with imatinib, as well as a paradoxical presentation of both occurring in the same patient.[4] Informing the patients about the possibility of these side effects can increase compliance to therapy. Further insight into the mechanisms of the pigmentary alterations caused by this drug is required for better treatment/prevention of these manifestations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Valizadeh N. Imatinib induced facial skin hyperpigmentation in a case of chronic myelogenous leukemia. Shiaz E Med J 2011;12:162-4.
[Google Scholar]
|
| 2. |
Liu LS, Colegio OR. Molecularly targeted therapies for melanoma. Int J Dermatol 2013;52:523-30.
[Google Scholar]
|
| 3. |
Song HS, Kang HY.Imatinib-mesylate induced hyperpigmentation of the nose and palate. Ann Dermatol 2014;26;532-3.
[Google Scholar]
|
| 4. |
Balagula Y, Pulitzer MP, Maki RG, Mysfowski PL. Pigmentary changes in a patient treated with imatinib. J Drugs Dermatol 2011;10:1062-6.
[Google Scholar]
|
| 5. |
Arora B, Kumar L, Sharma A, Wadhwa J, Kochupillai V. Pigmentary changes in chronic myeloid leukemia patients treated with imatinib mesylate. Ann Oncol 2004;15:358-9.
[Google Scholar]
|
| 6. |
Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse cutaneous reaction to imatinib (STI571) in Philadelphia chromosome – positive leukemias: A prospective study of 54 patients. J Am Acad Dermatol 2003;48:201-6.
[Google Scholar]
|
| 7. |
Prabhash K, Dovel DC. Lichenoid eruption due to imatinib. Indian J Dermatol Venereol Leprol 2005;71:287-8.
[Google Scholar]
|
| 8. |
Mcpherson T, Sherman V, Turner R. Imatinib-associated hyperpigmentation, a side effect that should be recognized. J Eur Acad Dermatol Venereol 2009;23:82-3.
[Google Scholar]
|
| 9. |
Mattson U, Halbritter S, Morner Serikoff E, Christerson L, Warfvinge G. Oral pigmentation in the hard palate associated with imatinib mesylate therapy: A report of three cases. Oral Surg Oral Med Pathol Oral Radiol Endod 2011;111:12-6.
[Google Scholar]
|
| 10. |
Song SH, Kang YH. Imatinib Mesylate-Induced Hyperpigmentation of the Nose and Palate. Ann Dermatol 2014;26:532-3.
[Google Scholar]
|
| 11. |
Kagimoto Y, Mizuashi M, Kikuchi K, Aiba S. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate. Int J Dermatol 2004;53:e161-2.
[Google Scholar]
|
| 12. |
Li CC, Malik SM, Blaeser BF, Dehni WJ, Kabani SP, Boyle N, et al. Mucosal pigmentation caused by imatinib: Report of three cases. Head Neck Pathol 2012;6:290-5.
[Google Scholar]
|
| 13. |
Han H, Yu YY, Wang YH. Imatinib mesylateeinduced repigmentation of vitiligo lesions in a patient with recurrent gastrointestinal stromal tumors. J Am Acad Dermatol 2008;59:S80-3.
[Google Scholar]
|
| 14. |
Alexandrescu DT, Dasanu CA, Ferzanmehr H, Kauffman L. Persistant cutaneous hyperpigmentation after tyrosine kinase inhibition with imatinib for GIST. Dermatol Online J 2008;14:7.
[Google Scholar]
|
| 15. |
Lapeere H, Boone B, Schepper SD, Verhaeghe E, Ongenae K, Geel NV, et al. Hypomelanosis and Hypermelanosis. Fitzpatrick's Dermatology in general medicine. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. 7th Ed. Mc Graw Hill, Boston; 2008. p. 635.
[Google Scholar]
|
Fulltext Views
5,669
PDF downloads
2,304





