Translate this page into:
Oseltamivir-induced toxic epidermal necrolysis in a patient with Cushing's disease
2 Department of Endocrinology and Nutrition, University Hospital Complex of Albacete, Albacete, Spain
3 PIELenRed Consortium, Príncipe de Asturias University Hospital, Alcalá de Henares, Madrid; Drug Hypersensitivity Laboratory, Institute for Health Research IdiPAZ, La Paz University Hospital, Albacete, Spain
4 PIELenRed Consortium, Príncipe de Asturias University Hospital, Alcalá de Henares; Department of Anatomical Pathology, La Paz University Hospital, Autonomous University of Madrid, Madrid, Spain
5 PIELenRed Consortium, Príncipe de Asturias University Hospital, Alcalá de Henares; Department of Clinical Pharmacology, La Paz University Hospital, Autonomous University of Madrid, Madrid, Spain
6 PIELenRed Consortium; Clinical Pharmacology Unit, Príncipe de Asturias University Hospital, Alcalá de Henares, Madrid, Spain
7 PIELenRed Consortium, Príncipe de Asturias University Hospital, Alcalá de Henares; Department of Endocrinology and Nutrition, La Paz University Hospital, Autonomous University of Madrid, Madrid, Spain
Correspondence Address:
J�ssica Gonz�lez-Ramos
La Paz University Hospital, Paseo La Castellana 261, Madrid CP 28046
Spain
| How to cite this article: Gonz�lez-Ramos J, Lamas C, Bell�n T, Ruiz-Bravo E, Ram�rez E, Lerma V, Lecumberri B. Oseltamivir-induced toxic epidermal necrolysis in a patient with Cushing's disease. Indian J Dermatol Venereol Leprol 2020;86:515-518 |
Abstract
We report a case of a patient with Cushing's disease with oseltamivir-induced toxic epidermal necrolysis, who was treated with cyclosporine with favorable evolution. There is only one case reported of Cushing's disease and toxic epidermal necrolysis and very few oseltamivir-induced toxic epidermal necrolysis cases in literature. This report also discusses the role that the preexisting hypercortisolism condition may have played in the development and favorable resolution of the toxic epidermal necrolysis.
Introduction
Toxic epidermal necrolysis or Lyell's syndrome and Stevens–Johnson syndrome are rare but severe drug-induced type IV delayed hypersensitivity reactions. Both are considered as severe variants of the same disease, where patients are classified as Stevens–Johnson syndrome, Stevens–Johnson syndrome/toxic epidermal necrolysis overlap or toxic epidermal necrolysis depending on the extension of cutaneous detachment. There is only one reported case of toxic epidermal necrolysis in a patient with Cushing's disease and two cases of Stevens–Johnson syndrome.[1],[2],[3] We report a case of a 43-year-old man diagnosed with Cushing's disease secondary to a pituitary microadenoma who, while waiting for surgical resection of the adenoma, started treatment with oseltamivir for influenza A and 18 days later he developed toxic epidermal necrolysis. All potentially culprit drugs were discontinued; the patient was transferred to a reference burn center where he received treatment with cyclosporine and highly specialized management and care, with favorable outcome.
Case Report
A 43-year-old man with no previous history of diseases was admitted to Albacete UniversityHospital Complex (Spain) on January 25, 2014, because of multiple osteoporotic spine and rib fractures. During hospitalization, a patent cushingoid phenotype was observed, and the patient was transferred to the department of Endocrinology. After an exhaustive study including catheterization of the inferior petrosal sinuses and sampling before and after corticotropin releasing hormone stimulation, the diagnosis of Cushing's disease secondary to a 5 mm microadenoma was confirmed. In addition, a refractory arterial hypertension and hypogonadotropic hypogonadism were diagnosed. Oseltamivir and meropenem were administered for 12 days because of influenza A virus infection, with improvement of the flu symptoms. Erythroderma appeared on February 23 (index day), rapidly evolving to epidermal detachment with positive Nikolsky sign, and more than, 30% of the body surface area affected [Figure - 1]a, [Figure - 1]b and [Figure - 1]c. Oral mucosa was involved, with absence of ocular or genital involvement. Clinical appearance of toxic epidermal necrolysis was similar to that of a patient without Cushing's disease. Skin biopsy confirmed the diagnosis of toxic epidermal necrolysis [Figure - 1]d. The SCORTEN (toxic epidermal necrolysis-specific severity-of-illness score) was 2.[4]
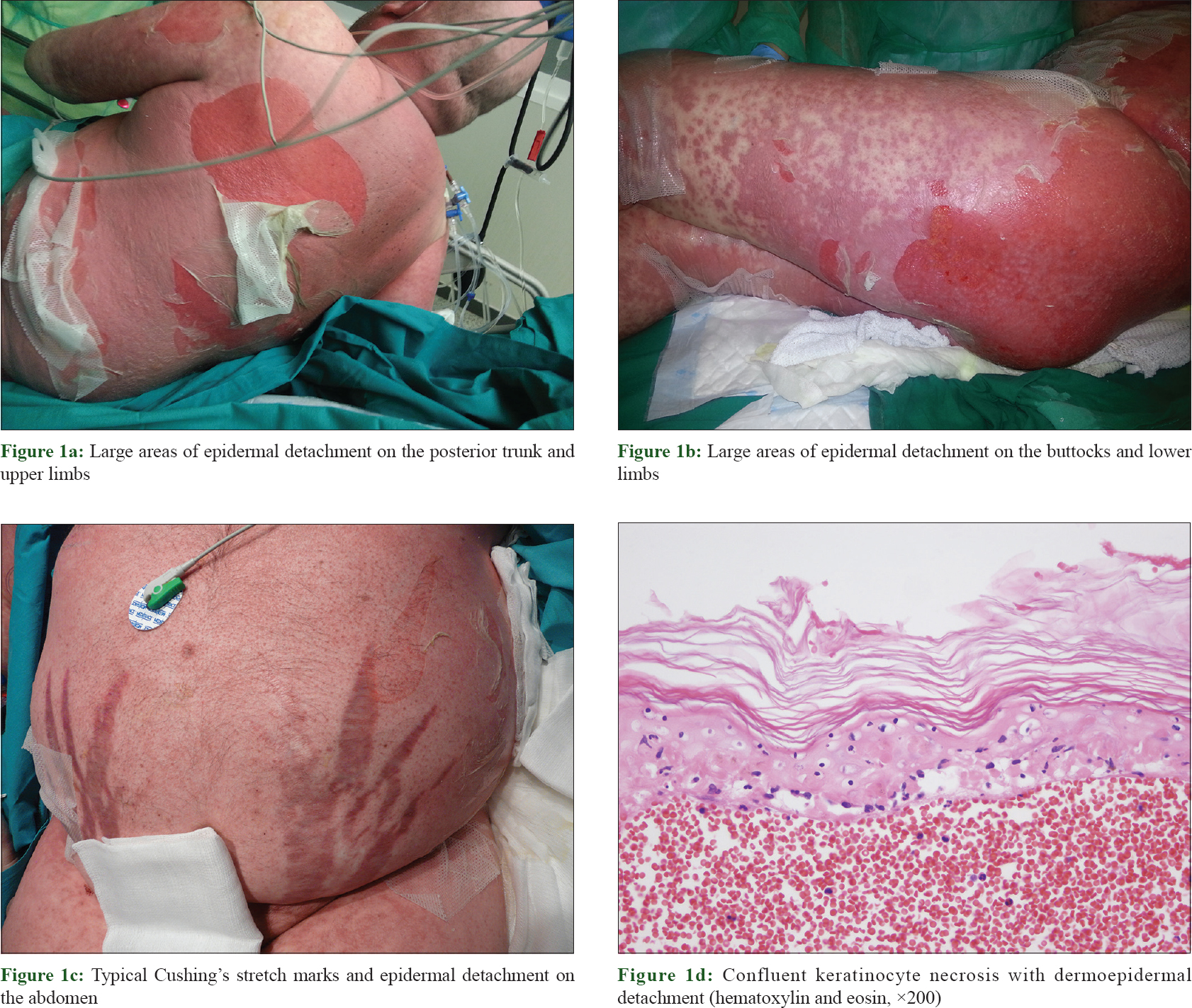 |
Ketoconazole was started 18 days before the onset of the toxic epidermal necrolysis symptoms, and teriparatide and testosterone 2 days before the onset of the symptoms [Table - 1]. After discontinuation of teriparatide, ketoconazole, oseltamivir and meropenem, the patient was transferred to La Paz University Hospital Burn Center (Madrid, Spain), where he was treated with intravenous cyclosporine 5 mg/kg/day. The dose was reduced to 3 mg/kg/day after 2 weeks, when re-epithelization was evident [Figure - 2], and stopped 4 weeks later when the patient was returned to his reference hospital.

 |
| Figure 2: Clinical appearance of lesions 2 weeks after starting treatment with cyclosporine |
During hospitalization a methicillin-resistant Staphylococcus aureus bacteremia and a severe vitamin D deficiency (6 ng/mL) were diagnosed. The bacteremia was treated with linezolid for 14 days, and the vitamin D deficiency with vitamin D supplements. The outcome was favorable in spite of the renal and respiratory impairments, with re-epithelialization starting 8 days after the onset of the disease and complete re-epithelialization 16 days after the onset. The microadenoma was later removed by transsphenoidal resection (April 4, 2014). After surgery no clinical or analytical symptoms related to Cushing's disease were observed.
A consultation was requested to the clinical pharmacology department, who performed the ALDEN algorithm for drug causality.[4] The scores were: 4+ for ketoconazole and 3+ for oseltamivir, meropenem, teriparatide and spironolactone. A lymphocyte transformation test was performed a year after the resolution of toxic epidermal necrolysis in order to confirm drug causality. Positive results were obtained with oseltamivir (stimulation index >2.5 in two independent assays) but were negative with ketoconazole and meropenem [Table - 2].

Discussion
The Stevens–Johnson syndrome/toxic epidermal necrolysis spectrum includes hypersensitivity reactions that typically occur 1–3 weeks after drug intake. Up to 15% of Stevens–Johnson syndrome/toxic epidermal necrolysis occurred in patients with chronic corticosteroid use. The pathophysiological manifestations are similar to those in burn patients, with frequent development of life-threatening systemic involvement (hepatic, respiratory, renal, etc.). The percentage of epidermal detachment is of prognostic value allowing the classification of patients in three groups: (a) Stevens–Johnson syndrome when < 10% of body surface area is involved; (b) Stevens–Johnson syndrome/toxic epidermal necrolysis overlap when 10–30% body surface area and (c) toxic epidermal necrolysis when body surface area is more than 30%. The incidence of toxic epidermal necrolysis ranges between 1.2 and 6 cases per million inhabitants per year. Mortality is predicted by the SCORTEN (range: 0–7 points). Stevens–Johnson syndrome/toxic epidermal necrolysis can be induced by any drug, but the most frequently involved are allopurinol, antiepileptic drugs, sulfonamides, antibiotics and nonsteroidal antiinflammatory drugs.[4] Occasionally vaccines, antiviral drugs, food additives, herbal medicines and viral or bacterial infections such as HIV, herpes simplex virus and Mycoplasma pneumoniae have also been reported as causative agents.[5]
The pathogenesis of toxic epidermal necrolysis is still incompletely understood. Available evidences indicate that the combination of genetic susceptibility, antigen-specific immunity and mediators of cell death play a key role in the mechanism of the disease. Toxic epidermal necrolysis is considered as a T-cell mediated type IV hypersensitivity disorder. CD8+ T cells and NK cells concentrate in blister fluid and epidermis of Stevens–Johnson syndrome/toxic epidermal necrolysis patients. In terms of apoptosis mediators, some studies observed that Fas/Fas ligand interactions participate in keratinocyte apoptosis induction, a critical feature of Stevens–Johnson syndrome/toxic epidermal necrolysis. Perforin/granzyme pathway is also involved in the keratinocyte apoptosis.
Oseltamivir is an antiviral drug used for treatment and prophylaxis of influenza A and B. Adverse events are usually mild; but some severe secondary effects have been reported. These are mainly neurologic or cutaneous adverse reactions, among which some toxic epidermal necrolysis cases have been described, one of them in a 1-year-old child.[5],[6]
Although the specific treatment for toxic epidermal necrolysis is still debated and has not yet been standardized, there is international consensus that supports early diagnosis, early withdrawal of the culprit drug and transfer to a specialized unit (preferably a burns unit) where specialized skin care and management have all proved to decrease mortality. Recent reports show that treatment with cyclosporine reduces mortality in toxic epidermal necrolysis patients.[4]
There is controversy about the use of systemic steroids, with some studies reporting an increase in the infection rate, delayed re-epithelialization and an increased mortality, although recent reports do not support these findings.[7] Pulse steroid therapy at early onset of the disease seems to reduce mortaliy[8] and in combination with intravenous immunoglobulin appears to reduce the time to recovery.[9] It has been reported that prior treatment with steroids delays the onset and progression of the disease but does not modify the severity or mortality.[10] In our patient it is difficult to know the role played by variations of cortisol levels in the disease; however, severe hypercortisolism was present before and after the onset of toxic epidermal necrolysis. This latter fact could have had a positive impact on the course of the disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Acknowledgements
The authors would like to thank all the physicians, nurses and nurse auxiliaries from La Paz University Hospital Burn Unit. The patient has been included in PIELenRed and RegiSCAR studies (patient code: 501-0111).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Pfliegler G, Udvardy M, Thomázy V, Vezekényi K, Alföldi G. Lyell syndrome in a patient with Cushing disease. Orv Hetil 1981;122:2781-3.
[Google Scholar]
|
| 2. |
Wammock VS, Biederman AA, Jordan RS. Erythema multiforme exudativum (Stevens-Johnson syndrome); report on a patient treated with pituitary adrenocorticotropic hormone. J Am Med Assoc 1951;147:637-9.
[Google Scholar]
|
| 3. |
Mustafa N, Periyasamy P, Kamaruddin N. Steven Johnson syndrome in a patient with Cushing's disease. Med J Malaysia 2009;64:238-9.
[Google Scholar]
|
| 4. |
Bellón T, Cabañas R, González-Herrada C, Ramírez E, González-Ramos J, López San Martín M, et al. Approach to severe cutaneous adverse drug reactions. Curr Treat Options Allergy 2017;4:201-21.
[Google Scholar]
|
| 5. |
Luna P, Zuazaga M, Chede C, Entin E, Larralde M. Toxic epidermal necrolysis after treatment with oseltamivir: Case report. Arch Argent Pediatr 2010;108:e76-8.
[Google Scholar]
|
| 6. |
Nordstrom BL, Oh K, Sacks ST, L'Italien GJ. Skin reactions in patients with influenza treated with oseltamivir: A retrospective cohort study. Antivir Ther 2004;9:187-95.
[Google Scholar]
|
| 7. |
Roongpisuthipong W, Prompongsa S, Klangjareonchai T. Retrospective analysis of corticosteroid treatment in Stevens-Johnson syndrome and/or toxic epidermal necrolysis over a period of 10 years in Vajira hospital, Navamindradhiraj University, Bangkok. Dermatol Res Pract 2014;2014:237821.
[Google Scholar]
|
| 8. |
Choonhakarn C, Limpawattana P, Chaowattanapanit S. Clinical profiles and treatment outcomes of systemic corticosteroids for toxic epidermal necrolysis: A retrospective study. J Dermatol 2016;43:156-61.
[Google Scholar]
|
| 9. |
Ye LP, Zhang C, Zhu QX. The effect of intravenous immunoglobulin combined with corticosteroid on the progression of Stevens-Johnson syndrome and toxic epidermal necrolysis: A meta-analysis. PLoS One 2016;11:e0167120.
[Google Scholar]
|
| 10. |
Lee HY, Dunant A, Sekula P, Mockenhaupt M, Wolkenstein P, Valeyrie-Allanore L, et al. The role of prior corticosteroid use on the clinical course of Stevens-Johnson syndrome and toxic epidermal necrolysis: A case-control analysis of patients selected from the multinational euroSCAR and regiSCAR studies. Br J Dermatol 2012;167:555-62.
[Google Scholar]
|
Fulltext Views
3,897
PDF downloads
2,375





