Translate this page into:
Rupioid psoriasis induced by pembrolizumab
Correspondence Address:
Jos� M Mascar�
Department of Dermatology, Hospital Clinic de Barcelona, University of Barcelona, 170, Villarroel Street, Barcelona 08036
Spain
| How to cite this article: Marti-Marti I, G�mez S, Riera-Monroig J, Carrera C, Mascar� JM. Rupioid psoriasis induced by pembrolizumab. Indian J Dermatol Venereol Leprol 2020;86:580-582 |
Sir,
Immune check point inhibitors are increasingly used in cancer treatment. Potential immune-related adverse events, notably mediated by the triggering of cytotoxic T cell activation, may occur. Dermatologic disorders can be triggered or exacerbated by these treatments. Here, we report a case of a rare case scenario of a patient developing a sudden flare of psoriasis after initiating treatment with pembrolizumab.[1]
A 67-year-old white man who had a history of mild plaque psoriasis since childhood controlled with on and off topical therapy, initiated treatment with a programmed cell death protein 1 inhibitor (pembrolizumab 200 mg every 3 weeks) for a metastatic squamous cell carcinoma of the lung. He had no active psoriatic lesions at that time and had been in remission for the last two years. Two months after initiating the treatment with pembrolizumab (after two cycles of medication), he presented to the emergency department with a two-week history of sudden development of hyperkeratotic erythematous plaques affecting his trunk, scalp, upper limbs and lower limbs that prevented him from walking.
On examination, the plaques were well-defined, round-shaped hyperkeratotic and erythematous with white, coarse and adherent scales [Figure - 1] and [Figure - 2], different from the psoriasis vulgaris lesions he previously had. He also had palmoplantar keratoderma with deep fissures [Figure - 3] and yellow discoloration of the nails with distal onycholysis. Laboratory testing was normal except leukocytosis of 15.0 × 10(9)/L and a C-reactive protein of 4.80 mg/dL and viral serology was negative. A clinical diagnosis of severe rupioid psoriasis was made. Use of the Naranjo Adverse Drug Reaction Probability Scale indicated a probable relationship (score = 5) between pembrolizumab and the cutaneous flare.[2] Anti-programmed cell death protein 1 therapy was temporary discontinued. Oral acitretin 25 mg/day and topical treatment with 10% salicylic acid and triamcinolone acetonide 0.1% were initiated with good response. Pembrolizumab was not reintroduced due to evidence of disease progression on the restaging positron emission tomography scan.
 |
| Figure 1: Hyperkeratotic plaques with coarse scales on the buttocks and oyster/limpet shell-shaped scales on the proximal lower limbs |
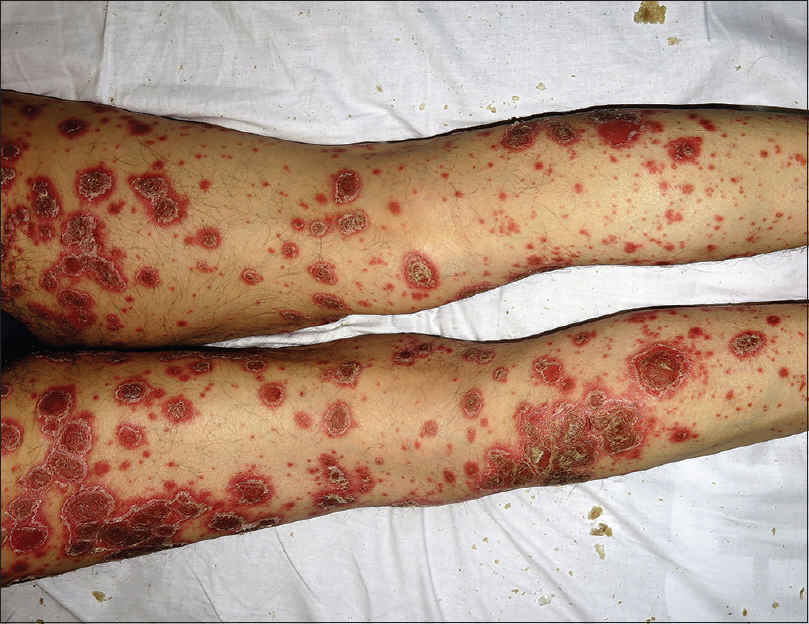 |
| Figure 2: Oyster/limpet shell shaped hyperkeratotic plaques on the dorsum of the lower limbs |
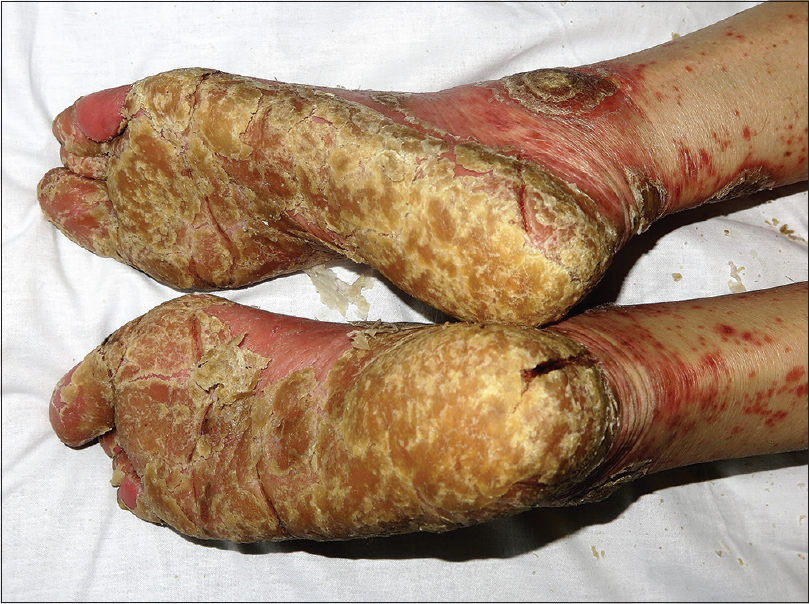 |
| Figure 3: Thick plantar keratoderma with severe fissures |
The term “ostraceous” or “rupioid” is used to describe well-demarcated, round-shaped hyperkeratotic plaques with thick scales resembling oyster or limpet shells, respectively. In addition to psoriasis, these lesions can also be observed in secondary syphilis, crusted (Norwegian) scabies and, rarely, in other diseases.[3]
Immune-check point inhibitors are a new class of drugs which could be associated with a new spectrum of adverse events. The most common cutaneous side effects are maculopapular rash (eczema-like spongiotic dermatitis) and pruritus.[4] However, many other cutaneous adverse effects may be observed, e.g., lichenoid muco-cutaneous reactions and vitiligo-like depigmentation.[1] Dermatologic evaluation is essential to detect sporadic conditions such as psoriasis, acneiform rash, rosacea, sarcoidosis, autoimmune bullous diseases, dermatomyositis, vasculitis, Sjogren's syndrome, alopecia, nail changes and neutrophilic dermatoses. It can even result in life-threatening adverse effects such as Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis and drug reaction with eosinophilia and systemic symptoms.[1]
As programmed cell death protein 1/ programmed death-ligand 1 inhibitors stimulate T-cell immune responses, they can trigger the development of “de novo” inflammatory skin diseases mediated by T-cells, such as psoriasis, or exacerbate other inflammatory skin conditions. It is widely accepted that activated T cells (Th1 and Th17) are the primary modulators in psoriasis. Immune-check point inhibitors act by enabling reemergence of T cells responses toward tumor neoantigens. However, this may also be associated with heightened reactivity against normal tissue leading to an exacerbation of psoriasis or other T cell mediated diseases.[5]
Cutaneous adverse effects are usually reported after the first couple of treatment cycles. Their onset is usually around 5 weeks with programmed cell death protein 1 inhibitors and 3-4 weeks with cytotoxic T-lymphocyte-associated protein 4 inhibitors. This time frame has been reported to be shorter if used in combination. The lesions can worsen after each cycle of treatment.[1] Some cases of severe psoriasis flares during such immunotherapy have been reported.[5] However, we were unable to find any previous reports on “rupioid presentation”.
Dermatologists should be aware that psoriasis and other dermatologic disorders can be triggered or exacerbated by immune checkpoint inhibitors and present with striking or atypical clinical presentations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol 2018;19:345-61.
[Google Scholar]
|
| 2. |
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45.
[Google Scholar]
|
| 3. |
Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis 2014;94:119-21.
[Google Scholar]
|
| 4. |
Gedye C, van der Westhuizen A, John T. Checkpoint immunotherapy for cancer: Superior survival, unaccustomed toxicities. Intern Med J 2015;45:696-701.
[Google Scholar]
|
| 5. |
Chia PL, John T. Severe psoriasis flare after anti-programmed death ligand 1 (PD-L1) therapy for metastatic non-small cell lung cancer (NSCLC). J Immunother 2016;39:202-4.
[Google Scholar]
|
Fulltext Views
5,907
PDF downloads
1,969





