Translate this page into:
Topical corticosteroids in dermatology
Correspondence Address:
Aayushi B Mehta
Dermatology OPD, D. Y. Patil School of Medicine, Sector 5, Nerul, Navi Mumbai - 400 706, Maharashtra
India
| How to cite this article: Mehta AB, Nadkarni NJ, Patil SP, Godse KV, Gautam M, Agarwal S. Topical corticosteroids in dermatology. Indian J Dermatol Venereol Leprol 2016;82:371-378 |
Abstract
Since their introduction, topical corticosteroids have become indispensable in the treatment of various dermatoses. Hydrocortisone was the first compound. Modifications in the basic structure generated in vivo activity and thus different topically active compounds were discovered. Apart from the Stoughton vasoconstrictor assay, various other methods are used for potency assessment of topical corticosteroids. Topical corticosteroides are classified based upon potency and action of these molecules. Mechanism of action at the cellular level and indications of topical corticosteroid use have been discussed. Various adverse effects often occur as an extension of their activity combined with inappropriate usage. Tachyphylaxis and contact allergy are potential problems in clinical practice. Newer compounds with improved risk-benefit ratio are available.Introduction
Topical corticosteroids act primarily by binding to glucocorticoid response elements in host DNA. They are effective in a number of dermatoses. Dermatotherapy has always been a combination of art and science. However, the science was rudimentary and mixed with empiricism and experience. Many current dermatological therapies had a serendipitous evolution. The modern era of dermatotherapy began with the introduction of topical corticosteroids. Since then, use of these agents has been manifold usually with remarkable but often temporary results.
History
Kendall et al. (1948) isolated various compounds from bovine adrenal glands and designated them with alphabets from A to F. Among these compound E (cortisone) and F (hydrocortisone) were found to be effective in human beings.[1] In 1952, Sulzberger and Witten reported the efficacy of topically applied compound F (hydrocortisone) in selected dermatoses, a landmark development in dermato-therapeutics.[2] Topical compound E (cortisone) was further found to be of no significant value in the treatment of skin disorders.[1]
Structure
Hydrocortisone is a natural glucocorticoid derived from the adrenal cortex. Its basic structure forms the backbone of most topical corticosteroid molecules. It exhibited much higher anti-inflammatory activity on topical application than did the parent compound, cortisone. Thus development of various topical corticosteroid compounds was initiated.[3]
Hydrocortisone is made up of 21 carbon atoms constituting the cyclo-pentano-perhydro- phenanthrene nucleus and a 17, 21-dihydroxy (OH)-20-keto (O) side chain [Figure - 1]. This side chain is crucial for the glucocorticoid effect.[4] The 4 rings in the structure are designated A to D. The hydroxyl (OH) group at C 11, the double bond at C4, 5 and the ketone moiety on C3 are also essential for glucocorticoid activity. This is the basic structure from which all other topical corticosteroid molecules are derived.[5]
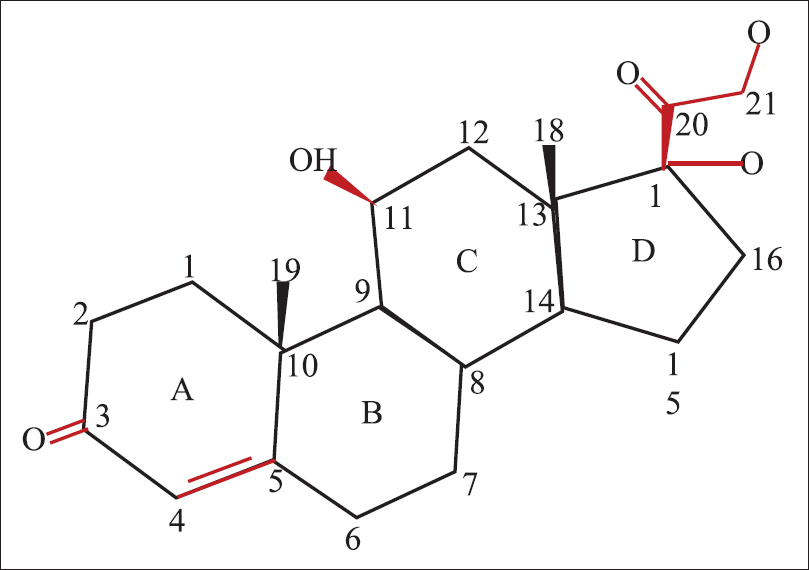 |
| Figure 1: Structure of hydrocortisone |
Fluorination at C6 and C9 atoms of this molecule increases potency while addition of an acetonide group enhances penetrability and percutaneous absorption.[3] Halogenation at C9 or C6 positions increases potency of the steroid, while simultaneous halogenation at both the carbons shows highest potency.[3],[6]Topical agents like triamcinolone and betamethasone possess a double bond at C1–C2 position with enhanced glucocorticoid activity and decreased rate of metabolism.[3]
The instrinsic potency of topical corticosteroids may be augmented by esterification and halogenation.[6] Topical corticosteroids can be classified as non-esters, mono-esters and di-esters on the basis of esterification including both halogenated and non-halogenated compounds in each class.[5] Esterification is done at 17- and 21-C positions.[6] This increases the lipophilicity and the 17–21 di-esters attain better penetrability through the skin.[6] Esterification with an acetate further improves this property.[3] Halogenation attributes higher mineralocorticoid property to the compound including anti-proliferative effect; this can be utilized as targeted therapy in conditions like psoriasis and chronic lichenified eczema.[6]
Metabolism
Intraepidermal de-esterification is one of the principal mechanisms of metabolism of topical corticosteroids. Earlier this was thought to be the explanation for tachyphylaxis seen with topical steroids but was subsequently disproved[7] Biotransformation of topical corticosteroids in the skin can be modulated advantageously to enhance potency.[6] Halogenation imparts resistance to de-esterification prolonging the active state of the compound (e.g. clobetasol). Formulations with increased affinity for intracellular corticosteroid receptors (e.g. methylprednisolone aceponate) have higher potency.
Potency Assessment
Human vasoconstrictor assay (McKenzie and Stoughton) is one of the most commonly used methods for assessing potency of topical corticosteroids.[8] It measures the degree of visible blanching caused by various dilutions of topical corticosteroids applied to human skin. This correlates well with clinical efficacy and outcome and thus forms the basis of the current classification system for topical corticosteroids.
The Dumas and Scholtz small plaque psoriasis bioassay is a modification of the vasoconstrictor assay and measures anti-inflammatory potency of topical corticosteroids.[9] Rat thymus involution assay and anti-granuloma assay are the other commonly used measures for anti-inflammatory property. Fibroblast assay is a measure of atrophogenicity.[10] The histamine pin-prick bioassay, first developed by Reddy and Singh,[11] is a simple and reliable procedure to assess the potency of corticosteroids. In spite of being a non-clinical assay, the mechanism of the assay resembles those involved in various dermatoses thus improving its applicability.
Delivery Vehicles
Several formulations of topical corticosteroids are available, including ointments, creams, gels, lotions, solutions and newer formulations such as shampoos and foams. Multiple factors influence the choice of formulation for individual patients.[12],[13] Ointments have higher penetrability and are useful for thickened skin over palms and soles and over lichenified skin. They are relatively greasy and messy to use. Creams are less greasy and are more suited for moist and weeping areas of skin. Gels are non-greasy, non-occlusive and may cause local stinging and irritation. They are useful for hairy areas and face as they have the advantage of leaving less residue. Lotions spread easily and are useful for large surface areas, hairy areas and skin flexures. Solutions are alcohol based formulations which are also useful for hairy and intertriginous areas. Foams are newer formulations. They are pressurized liquids packed with a propellant which form a liquid/semi-solid product upon actuation.[13] They are non-messy and mainly useful on the scalp. Shampoos are also available for use on the scalp.
Vehicles and formulation play a very important role in determining the potency of topical corticosteroids.[3] Fluocinonide, when it was first developed, exhibited superior activity in biological tests. However, when it was compounded into the standard cream and vehicle formulations, it did not demonstrate the same activity. The currently used vehicle has overcome this difficulty achieving its category II potency.[3]
Mechanism of Action
Topical corticosteroids exert biological effects by binding to the glucocorticoid receptor. The receptor is cytoplasmic in the unbound state and is translocated to the nucleus after glucocorticoid binding.[14] [Figure - 2] illustrates the mechanism of action of glucocorticoids. Binding to glucocorticoid response elements in host DNA represents the most important pathway for most glucocorticoid actions. Cytoplasmic interaction of the glucocorticoid receptor with cellular transcription proteins is the other proposed mechanism of action.[15]
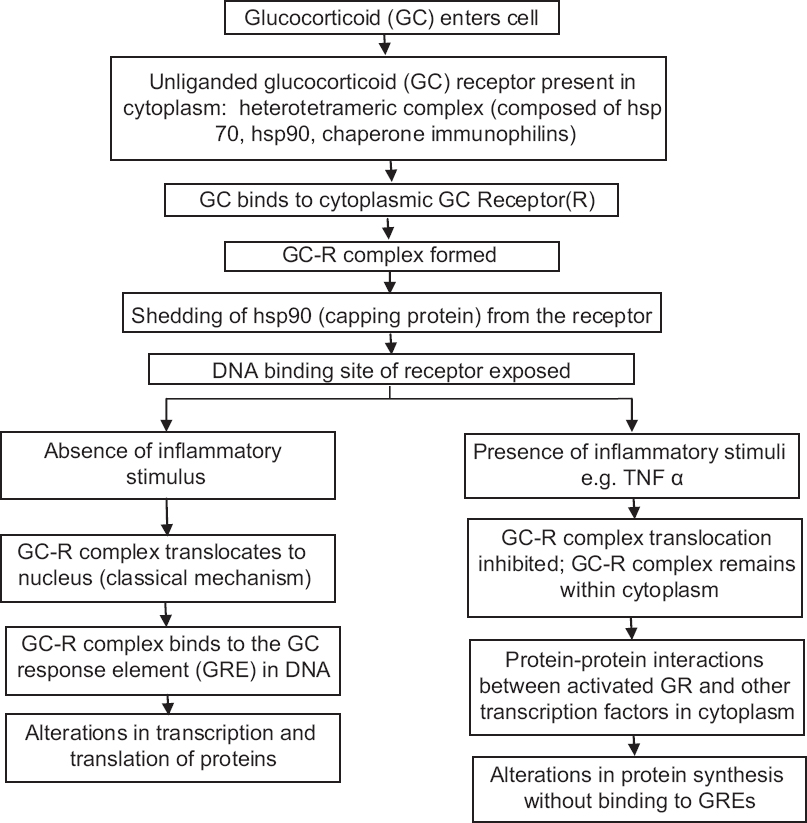 |
| Figure 2: Proposed mechanism of action of topical corticosteroids |
Classification
As per the currently used potency-based classification system, topical corticosteroids can be divided into 7 classes,[16] Class I superpotent (clobetasol propionate 0.05%, halobetasol propionate 0.05%, desoximetasone 0.25%), Class II: high-potent (betamethasone dipropionate 0.05% cream, halcinonide 0.1%), Class III: medium-high potency (fluticasone propionate 0.005% ointment), Class IV medium potency (mometasone furoate 0.1% cream), Class V: medium potency (betamethasone valerate 0.1% cream, fluocinolone acetonide 0.025% cream), Class VI: low potency (desonide 0.05% cream, fluocinolone acetonide 0.01% cream), and Class VII: low potency (hydrocortisone acetate, dexamethasone acetate 0.1%).
Non-halogenated corticosteroids are hydrocortisone (and its derivatives), desonide and prednicarbate. Methylprednisolone aceponate is a newer, non-halogenated topical corticosteroid, but is not yet available in India.
Indications
In highly steroid responsive dermatoses, use of low to medium potency corticosteroids is sufficient to induce rapid remission. In less-responsive disorders, corticosteroids with higher potency may be used with or without occlusion to an achieve an optimal clinical response. In poorly responsive disorders, use of super potent or intralesional corticosteroids is often required.[17] Dermatoses categorized according to responsiveness to topical corticosteroids are presented in [Table - 1].
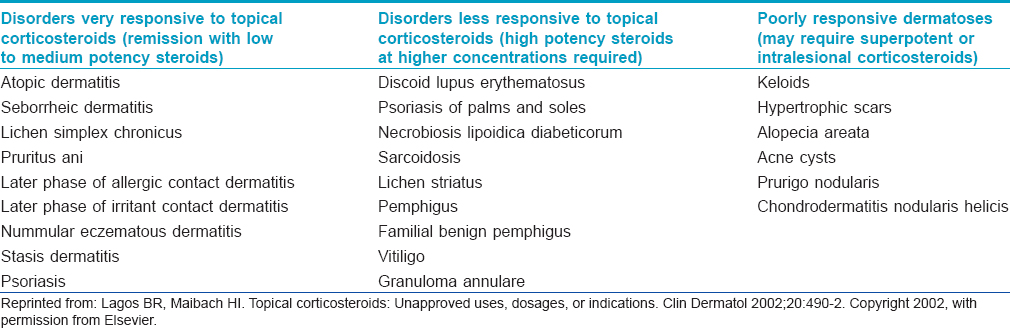
Topical corticosteroids constitute the first line therapy in conditions like eczema and often cannot be replaced by other compounds. The success rate of treatment of localized vitiligo has been shown to be highest with topical corticosteroids.[18] In various studies, topical clobetasol has been found to be significantly more effective than tacrolimus in the treatment of vulvar lichen sclerosus.[19]
Pregnancy Category
All topical corticosteroids are included under pregnancy category C (benefit outweighs risk) and these should be prescribed with caution during lactation.[16]
A Cochrane review in 2009 found that there was not enough data to establish correlation between topical corticosteroid use during pregnancy and congenital abnormalities, preterm delivery or still birth.[20] Use of very potent topical corticosteroids during pregnancy may have the risk of low birth weight babies. However, current evidence for this observation is very low and further studies may validate the above observations.[20]
Choice of Corticosteroid, Application Technique and Frequency
Various factors affecting the choice of topical corticosteroids and application frequency have been enumerated in [Table - 2]. In body sites with thin skin (face, eyelids, scrotum and flexures), milder corticosteroids should be used and also in dermatoses involving extensive body surface areas and in children; such practice is meant to reduce therapy-related side effects.
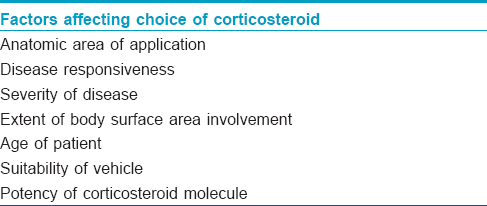
Topically applied corticosteroids undergo slow absorption probably attributed to “the reservoir effect.” [17],[21] This may influence the decision on frequency of topical application. Some clinical trials have shown no difference in efficacy if these drugs are used once daily rather than several times. A recently published study showed that the reservoir effect of certain corticosteroids may persist for up to 5 days.[21] Thus, the ideal frequency of application needs to be re-evaluated and validated by further research.[21]
A standardized 'fingertip unit (FTU)' and 'rule of hand' have been devised by Long and Finlay to measure the amount of ointment necessary to cover specific anatomic areas adequately.[22],[23] A 'fingertip unit' is the amount of ointment expressed from a tube (nozzle diameter 5 mm) applied from the distal skin crease to the tip of the palmar aspect of the index finger.[22] The usual number of fingertip units required for adequate coverage of specific body sites in adults has been presented in [Table - 3]. The 'rule of hand' states that the area of one side of a flat closed hand requires approximately 0.5 fingertip units or 0.25 g of ointment.[23]
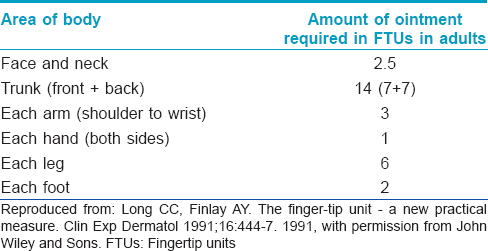
One fingertip unit is approximately equivalent to 0.49 g and 0.43 g of ointment by weight for males and females respectively.[22] Hence, approximately 282 g of ointment would be required for twice daily application to the total body surface (except scalp) for 1 week in an adult male.[22] The inter-relation between 'rule of hand' and 'finger tip unit' is as given below:
“4 hand areas = 2 fingertip units = 1 g ointment.”
This is a simple and useful way to counsel patients regarding the correct amount of topical steroid to be used.
Duration of Use
It has been recommended that superpotent topical corticosteroids such as clobetasol and halobetasol propionate not be used for a duration greater than 14 days with the total dose not exceeding 50 gms per week for halobetasol and 60 gms per week for clobetasol.[24] Use under occlusion of these compounds is not recommended. Usage of betamethasone dipropionate should not exceed 45 gms per week.[24] There are no guidelines for other classes but the general recommendation is to switch over to a corticosteroid of lower potency or non-steroidal preparations for maintenance therapy after 2 weeks.
Use of Soaks and Wet-Wraps
Wet-wrap therapy is a technique where topical corticosteroids are applied followed by wet gauze or cotton pads being rolled over affected body areas with the aim of enhancing penetration and efficacy. Use of wet-wrap with diluted topical corticosteroids has been found to be more efficacious as a short-term intervention than only emollients in children with atopic dermatitis.[25] A recent review concludes that wet-wrap therapy is also a useful therapeutic modality for conditions other than atopic dermatitis such as eczemas, psoriasis and prurigo nodularis. Though half of the studies reviewed by the authors showed a better therapeutic response with this modality, this observation was not comparable to other study results because of disparity in methodologies.[26]
Tachyphylaxis
Topical corticosteroids are susceptible to develop tachyphylaxis. Tachyphylaxis (acute tolerance) is defined as a rapidly diminishing response to successive doses of a drug rendering it less effective. Clinical trials studying the phenomenon of tachyphylaxis are lacking.[27]
It is now proposed that the mechanism of action of topical corticosteroids in psoriasis is not related to mechanisms which lead to development of tolerance, rather it is the immunosuppressive effect.[27] Some studies on topical corticosteroid therapy in patients with psoriasis have not demonstrated tachyphylaxis.[28],[29] Some authors have proposed that poor patient compliance to topical therapy over time may be perceived as tachyphylaxis rather than actual reduction in drug efficacy.[30]
In a systematic review by Taheri et al. two theories on development of resistance to topical corticosteroids and relapse of diseases have been proposed.[27] The first is non-adherence to therapy by patients in the long run. The second postulation is that there is an initial maximal response during the first few weeks of therapy followed by a plateau. During this plateau period, corticosteroids have limited effectiveness. However, natural disease flare-ups during this period may give the wrong impression of tachyphylaxis. The authors have also proposed that when this unresponsiveness is observed in patients on long-term topical corticosteroid therapy the term 'bradyphylaxis' may be used[27] it has been defined as “a slow, progressive, reduction in response to treatment over prolonged periods of use.” [31]
Side Effects[32],[33],[34],[35]
Various adverse effects occuring due to topical corticosteroids have been extensively published in the literature. They can be broadly classified as local and systemic adverse effects. The immediate effects include stinging and irritation. Effects on the epidermis are atrophy, hypo/hyperpigmentation, photosensitivity, loss of skin barrier and premature aging.
Topical steroids also adversely affect dermal functions such as wound healing and collagen formation leading to telengiectasias, ulcerations, delayed wound healing, striae distensae, Bateman's purpura, easy bruising and stellate scars. Steroid-induced acne, rosacea, hypertrichosis and alopecia are known adverse events.
Steroids also increase susceptibility to infections[33],[35] leading to altered presentations such as scabies incognito/crusted scabies, candidiasis, tinea incognito, herpes incognito, impetigo incognito and demodicidiosis. Steroids can also cause allergic contact dermatitis, perioral dermatitis, contact urticaria and granuloma gluteale infantum. Topical steroid- induced rosacea and acne are frequently seen in practice.
Withdrawal of steroids frequently leads to rebound flares of psoriasis, pustular psoriasis, reactivation of Kaposi's sarcoma and rebound erythema of the face,
Topical steroid damaged facies is a newly described entity associated with topical corticosteroid abuse.[33] Steroid addiction, red burning skin syndrome and status cosmeticus have been reported in the literature.
Systemic side effects of topical steroids include hypothalamo-pituitary-adrenal axis suppression, new onset diabetes mellitus/hyperglycaemia, iatrogenic Cushing's syndrome, mineralocorticoid effects (edema, hypocalcemia, hypokalemia, hypertension) and adrenal suppression.[36],[37],[38]
Steroids can also induce growth retardation in children, osteroporosis and avascular necrosis of bones in adults. Ocular side effects include posterior subcapsular cataract and glaucoma.[33]
Proper patient selection, careful and correct prescription, appropriate use of the drug and adequate counseling remain the mainstay of preventing adverse effects of topical steroids. Some specific ways to reduce the incidence of side effects are use of steroids with lower potency in susceptible age groups, tapering of doses and frequency of application after initial therapeutic response is achieved, week-end maintenance therapy, once daily applications and avoidance of occlusion.[32],[33]
Contact Allergy to Topical Corticosteroids
With the introduction of newer topical corticosteroid molecules and formulations, contact allergy has become an increasing problem in dermatological practice. More cases are now being recognized because of increasing awareness of the entity.[39] Prevalence of positive contact allergy to topical corticosteroids is between 0.2% to 6%.[32]
Contact allergy should be suspected in a case with worsening of symptoms or lack of expected improvement in conditions otherwise responsive to topical corticosteroids. Pathogenesis of contact allergy due to topical corticosteroid use has been depicted in [Figure - 3]. The inciting factor may be any component of the formulation, either the excipients or the corticosteroid molecule itself.[39] Allergens from the package may also be contributory.[39] Binding to the aminoacid arginine in the skin has been proposed to be a prerequisite for the development of contact allergy.[32],[39]
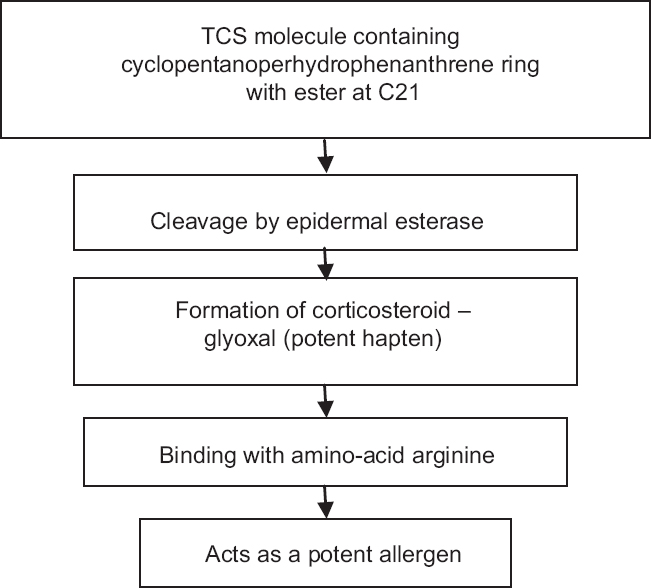 |
| Figure 3: Pathogenesis of contact allergy to topical corticosteroids. Adapted with permission from: Saraswat A. Contact allergy to topical corticosteroids and sunscreens. Indian J Dermatol Venereol Leprol 2012;78:552-9 |
Non-fluorinated corticosteroids have been found to have a higher prevalence of contact allergy as compared to fluorinated compounds.[32] Halogenation stabilizes the corticosteroid molecule and renders it less liable to degradation and possible sensitization.[39]
A classification system based on the structural features of topical corticosteroid compounds has been devised to predict cross-sensitivity on patch testing. This consists of four groups; namely, group A (hydrocortisone type), group B (triamcinolone acetonide type), group C (betamethasone type) and group D (hydrocortisone butyrate type).[40]
Topical Corticosteroids with an Improved Risk-Benefit Ratio
Chemical modification of the conventional glucocorticoid molecule has improved the risk-benefit ratio of these drugs.
Insertion of a halogen atom at 21-C position improves the stability of topical corticosteroid molecules; however, it also enhances the unwanted mineralocorticoid effects.[6] Thus, most of the glucocorticoids with improved risk-benefit ratio are non-halogenated double esters.[41] These include compounds such as prednicarbate, methylprednisolone aceponate, mometasone furoate and hydrocortisone aceponate.[42]
Prednicarbate is a non-halogenated double ester molecule with low atrophogenicity derived from prednisolone. While it acts against IL-1α in keratinocytes causing an anti-inflammatory effect, it does not affect fibroblasts thus lowering the atrophogenic potential. It can be used in children, the elderly and for long term intermittent use.[43] Fluticasone propionate is a potent topical corticosteroid with good anti-inflammatory activity and low potential for atrophy and adrenal suppression. Hypersensitivity and cross-sensitivity reactions with this compound are also very rare.[42] Mometasone furoate is a synthetic steroid with highly effective anti-inflammatory effect (equivalent to betamethasone) but only half the potential to cause HPA axis suppression. It has the additional advantage of once daily application.[42] Methylprednisolone aceponate is a newer non-halogenated molecule which has strong anti-inflammatory activity, weak atrophogenicity and no significant effect on plasma cortisol.[42]
Conclusion
Even several decades after their introduction, topical corticosteroids are first line therapy for various dermatological conditions. Though topical calcineurin inhibitors have become available, the value of topical corticosteroids has not diminished. However, maintaining the fine balance between judicious use and frequent abuse of these compounds is difficult. Until newer and safer topical corticosteroids become available, physician vigilance and patient education remain the mainstay of tackling this growing problem.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Cooney WP 3rd. Compound F: The history of hydrocortisone and hand surgery. J Hand Surg Am 2013;38:774-8.
[Google Scholar]
|
| 2. |
Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol 1952;19:101-2.
[Google Scholar]
|
| 3. |
Katz M, Gans EH. Topical corticosteroids, structure-activity and the glucocorticoid receptor: Discovery and development – A process of “planned serendipity” . J Pharm Sci 2008;97:2936-47.
[Google Scholar]
|
| 4. |
Rousseau GG, Schmit JP. Structure-activity relationships for glucocorticoids-I. Determination of receptor binding and biological activity. J Steroid Biochem 1977;8:911-9.
[Google Scholar]
|
| 5. |
Burkholder B. Topical corticosteroids: An update. Curr Probl Dermatol 2000;12:222-5.
[Google Scholar]
|
| 6. |
Mori M, Pimpinelli N, Giannotti B. Topical corticosteroids and unwanted local effects. Improving the benefit/risk ratio. Drug Saf 1994;10:406-12.
[Google Scholar]
|
| 7. |
Silva CM, Powell-Oliver FE, Jewell CM, Sar M, Allgood VE, Cidlowski JA. Regulation of the human glucocorticoid receptor by long-term and chronic treatment with glucocorticoid. Steroids 1994;59:436-42.
[Google Scholar]
|
| 8. |
McKenzie AW, Stoughton RB. Method for comparing percutaneous absorption of steroids. Arch Dermatol 1962;86:608-10.
[Google Scholar]
|
| 9. |
Dumas KJ, Scholtz JR. The psoriasis bio-assay for topical corticosteroid activity. Acta Derm Venereol 1972;52:43-8.
[Google Scholar]
|
| 10. |
Yohn JJ, Weston WL. Topical glucocorticosteroids. Curr Probl Dermatol 1990;2:38-63.
[Google Scholar]
|
| 11. |
Reddy BS, Singh G. A new model for human bioassay of topical corticosteroids. Br J Dermatol 1976;94:191-3.
[Google Scholar]
|
| 12. |
Kurian A, Barankin B. Delivery vehicle advances in dermatology. Fam Pract Ed 2011;7:4-5.
[Google Scholar]
|
| 13. |
Rosen J, Landriscina A, Friedman AJ. Principles and approaches for optimizing therapy with unique topical vehicles. J Drugs Dermatol 2014;13:1431-5.
[Google Scholar]
|
| 14. |
Ahluwalia A. Topical glucocorticoids and the skin – Mechanisms of action: An update. Mediators Inflamm 1998;7:183-93.
[Google Scholar]
|
| 15. |
Adcock IM, Brown CR, Barnes PJ. Tumour necrosis factor alpha causes retention of activated glucocorticoid receptor within the cytoplasm of A549 cells. Biochem Biophys Res Commun 1996;225:545-50.
[Google Scholar]
|
| 16. |
Tadicherla S, Ross K, Shenefelt PD, Fenske NA. Topical corticosteroids in dermatology. J Drugs Dermatol 2009;8:1093-105.
[Google Scholar]
|
| 17. |
Lagos BR, Maibach HI. Topical corticosteroids: Unapproved uses, dosages, or indications. Clin Dermatol 2002;20:490-2.
[Google Scholar]
|
| 18. |
Tamesis ME, Morelli JG. Vitiligo treatment in childhood: A state of the art review. Pediatr Dermatol 2010;27:437-45.
[Google Scholar]
|
| 19. |
Funaro D, Lovett A, Leroux N, Powell J. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J Am Acad Dermatol 2014;71:84-91.
[Google Scholar]
|
| 20. |
Chi CC, Lee CW, Wojnarowska F, Kirtschig G. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev 2009;(8):CD007346.
[Google Scholar]
|
| 21. |
Singh SK, Nasir F. The reservoir effect of topical steroids in vitiliginous skin: A cross-sectional study. Indian J Dermatol Venereol Leprol 2015;81:370-5.
[Google Scholar]
|
| 22. |
Long CC, Finlay AY. The finger-tip unit – A new practical measure. Clin Exp Dermatol 1991;16:444-7.
[Google Scholar]
|
| 23. |
Long CC, Finlay AY, Averill RW. The rule of hand: 4 hand areas=2 FTU=1 g. Arch Dermatol 1992;128:1129-30.
[Google Scholar]
|
| 24. |
Habif TP, editor. Topical therapy and topical corticosteroids. In: Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. China: Elsevier Inc.; 2010. p. 75-90.
[Google Scholar]
|
| 25. |
Devillers AC, Oranje AP. Efficacy and safety of 'wet-wrap' dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: A critical review of the literature. Br J Dermatol 2006;154:579-85.
[Google Scholar]
|
| 26. |
Andersen RM, Thyssen JP, Maibach HI. The role of wet wrap therapy in skin disorders – A literature review. Acta Derm Venereol 2015; May 5. doi: 10.2340/00015555-3134. [Epub ahead of print]
[Google Scholar]
|
| 27. |
Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids; what is the evidence? Dermatol Online J 2013;19:18954.
[Google Scholar]
|
| 28. |
Miller JJ, Roling D, Margolis D, Guzzo C. Failure to demonstrate therapeutic tachyphylaxis to topically applied steroids in patients with psoriasis. J Am Acad Dermatol 1999;41:546-9.
[Google Scholar]
|
| 29. |
Czarnowicki T, Linkner RV, Suárez-Fariñas M, Ingber A, Lebwohl M. An investigator-initiated, double-blind, vehicle-controlled pilot study: Assessment for tachyphylaxis to topically occluded halobetasol 0.05% ointment in the treatment of psoriasis. J Am Acad Dermatol 2014;71:954-959.e1.
[Google Scholar]
|
| 30. |
Feldman SR. Tachyphylaxis to topical corticosteroids: The more you use them, the less they work? Clin Dermatol 2006;24:229-30.
[Google Scholar]
|
| 31. |
Jefferson JW. It looks like “bradyphylaxis” to me. J Clin Psychiatry 2005;66:1076.
[Google Scholar]
|
| 32. |
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006;54:1-15.
[Google Scholar]
|
| 33. |
Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol Online J 2014;5:416-25.
[Google Scholar]
|
| 34. |
Fisher DA. Adverse effects of topical corticosteroid use. West J Med 1995;162:123-6.
[Google Scholar]
|
| 35. |
Abraham A, Roga G. Topical steroid-damaged skin. Indian J Dermatol 2014;59:456-9.
[Google Scholar]
|
| 36. |
Böckle BC, Jara D, Nindl W, Aberer W, Sepp NT. Adrenal insufficiency as a result of long-term misuse of topical corticosteroids. Dermatology 2014;228:289-93.
[Google Scholar]
|
| 37. |
van der Linden MW, Penning-van Beest FJ, Nijsten T, Herings RM. Topical corticosteroids and the risk of diabetes mellitus: A nested case-control study in the Netherlands. Drug Saf 2009;32:527-37.
[Google Scholar]
|
| 38. |
Dhar S, Seth J, Parikh D. Systemic side-effects of topical corticosteroids. Indian J Dermatol 2014;59:460-4.
[Google Scholar]
|
| 39. |
Saraswat A. Contact allergy to topical corticosteroids and sunscreens. Indian J Dermatol Venereol Leprol 2012;78:552-9.
[Google Scholar]
|
| 40. |
Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol 1989;121:27-34.
[Google Scholar]
|
| 41. |
Schäfer-Korting M, Schmid MH, Korting HC. Topical glucocorticoids with improved risk-benefit ratio. Rationale of a new concept. Drug Saf 1996;14:375-85.
[Google Scholar]
|
| 42. |
Brazzini B, Pimpinelli N. New and established topical corticosteroids in dermatology: Clinical pharmacology and therapeutic use. Am J Clin Dermatol 2002;3(1):47-58.
[Google Scholar]
|
| 43. |
Gupta AK, Chow M. Prednicarbate (Dermatop): Profile of a corticosteroid. J Cutan Med Surg. 2004;8:244-7.
[Google Scholar]
|
Fulltext Views
92,345
PDF downloads
14,033





