Translate this page into:
Polymorphism of glutathione S-transferase M1 and T1 genes and susceptibility to psoriasis disease: A study from North India
2 Department of Skin and VD, Pt. B.D. Sharma PGIMS, Rohtak, Haryana, India
3 Department of Genetics, M.D. University, Rohtak, Haryana, India
Correspondence Address:
Vijay K Jain
Department of Skin and VD, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, Haryana - 124 001
India
| How to cite this article: Srivastava DS, Jain VK, Verma P, Yadav JP. Polymorphism of glutathione S-transferase M1 and T1 genes and susceptibility to psoriasis disease: A study from North India. Indian J Dermatol Venereol Leprol 2018;84:39-44 |
Abstract
Background: Increased oxidative stress and resulting inflammation has been emphasized as a factor in the pathogenesis of many diseases including psoriasis. Glutathione S-transferases (GSTs) protect against oxidative stress, inflammation, and genotoxicity. Polymorphisms in the GST genes may lead to an imbalance in pro- and antioxidant systems resulting in the increased production of reactive oxygen species that could influence the pathogenesis of psoriasis.Aim: The aim of this study was to investigate the association between GSTs (GSTM1 and GSTT1) gene polymorphism in patients with chronic plaque psoriasis as a factor in the susceptibility and development of psoriasis.
Materials and Methods: We assessed 128 patients with psoriasis and 250 age- and sex-matched healthy controls. Genomic DNA was extracted from peripheral blood by the phenol chloroform method. The null GSTT1 and GSTM1 genotypes were identified by multiplex polymerase chain reaction (PCR) method.
Results: The null genotype of GSTM1 and GSTT1 was seen in 45.3% and 40.6% in psoriasis patients whereas in the controls it was 34.4% and 20.0%, respectively. A significant association was seen between the null alleles of the GSTT1 (OR = 2.74) and GSTM1 (OR = 1.58) alone or in combination with tobacco use (P < 0.001) and psoriasis risk. The presence of both null genotypes of GSTM1 and GSTT1 further increased the risk of psoriasis (OR = 3.52) when compared with the positive genotypes of GSTM1 and GSTT1.
Limitations: A major limitation of this study was the small sample size. A large epidemiological study is necessary to confirm these findings.
Conclusions: The null genotype of GSTT1 is a strong predisposing factor for psoriasis in North India.
Introduction
Psoriasis is a chronic, inflammatory, hyper-proliferative cutaneous disorder affecting 2–3% of the world population.[1] Although the exact pathogenesis of psoriasis is still unclear, there is evidence that oxidative stress, genetic predisposition, infections, physical trauma, medications, and environmental factors may influence psoriasis either individually or in concert.[2],[3],[4],[5],[6],[7],[8] Environmental toxins such as polycyclic aromatic hydrocarbons (PAHs) and hydroxylated metabolites of benzo (a) pyrene (xenobiotics) may influence the development of psoriasis. These chemicals can generate reactive oxygen species leading to oxidative damage of skin cells.[4],[5],[8],[9],[10] Between 20–90% of the variability in disposition of xenobiotics has been attributed to genetic factors.[3],[7] The genotype-specific form of drug metabolizing enzymes play an important role in the biotransformation of endogenous or exogenous compounds and might be associated in psoriasis.[4],[5],[8],[9],[11],[12]
Published data suggest that increased activity of antioxidant enzymes have synergistic effects in the reduction of oxidative damage and have role in cell protection.[13],[14],[15],[16] Metabolism of xenobiotics involves bioactivation by phase I enzymes (cytochrome p450s) resulting in the production of metabolites that may react with DNA. Phase II enzymes such as glutathione S-transferases (GSTs), are involved in the detoxification process of xenobiotics. GSTs are a family of related isozymes that catalyze the conjugation of reduced glutathione to a wide range of electrophilic substrates and protect against oxidative stress, inflammation, mutagenicity and genotoxicity.[17] Polymorphisms of specific subtypes of GST enzymes might lead to an imbalance in the pro- and antioxidant system. The most extensively studied polymorphisms are the GSTM1 null, GSTT1 null, and the GSTP1313 A/G substitution that are linked to decreased conjugation of biologically active xenobiotic metabolites. The functional consequences of the GSTM1 and the GSTT1 null genotype frequencies vary according to nationality and ethnicity.[18],[19]
Recent studies have shown that the presence of null genotypes of GSTM1 and GSTT1 enzymes are associated with increased susceptibility to several diseases including psoriasis and vitiligo.[20],[21],[22],[23],[24] Although some studies of GSTM1 and GSTT1 gene polymorphism and susceptibility to psoriasis have been reported,[9],[22],[25],[26] there are no reports from India. The current study was undertaken to ascertain whether high-risk alleles of GSTM1/GSTT1 could influence the susceptibility to psoriasis in the North Indian population. We also aimed to assess whether these alleles could affect the grade and duration of psoriasis, and their relationship with tobacco-use.
Materials and Methods
Subjects
Approval for the study was obtained from the institutional review board. The study group consisted of 128 psoriasis patients with a mean age 41.9 years (SE 1.48), and 250 age and sex-matched normal healthy individuals as controls with a mean age of 42.9 years (SE 0.57). The ethnic origin of the cases and controls were similar. Patients were selected based on the basis of a questionnaire administered in the Outpatient Department (OPD) of the Department Of Skin and VD, Pt. B.D. Sharma PGIMS, Rohtak, that included medical records, family history of disease, gender, and history of consumption of tobacco in any form (cigarette/bidi smoking, chewing tobacco in beetle leaf, pan masala/gutka, etc.). Only patients with uncomplicated chronic plaque psoriasis were selected. Psoriasis patients with associated conditions such as diabetes, bronchial asthma, cancer, or any other diseases were excluded from the study. Consent from the participants was obtained after explaining the aims of the study, and age- and sex-matched healthy individuals were selected as controls. The inclusion criteria for controls were the absence of any prior history of psoriasis lesions.
DNA extraction and genotyping
Five ml of blood was collected in EDTA vials from controls and patients. Genomic DNA was extracted from blood lymphocytes using the proteinase K and phenol chloroform extraction procedure.[27] The multiplex PCR method was used to detect the presence or absence of GSTT1 and GSTM1 genes in the genomic DNA samples, simultaneously in the same tube, as described previously.[28] Electrophoreses of PCR products were done in 2% agarose gels and visualized by ethidium bromide staining. DNA from samples positive for GSTM1 and GSTT1 genotypes yielded bands of 215 bp and 480 bp whereas internal positive control (CYP1A1) PCR product corresponded to 312 bp [Figure - 1].
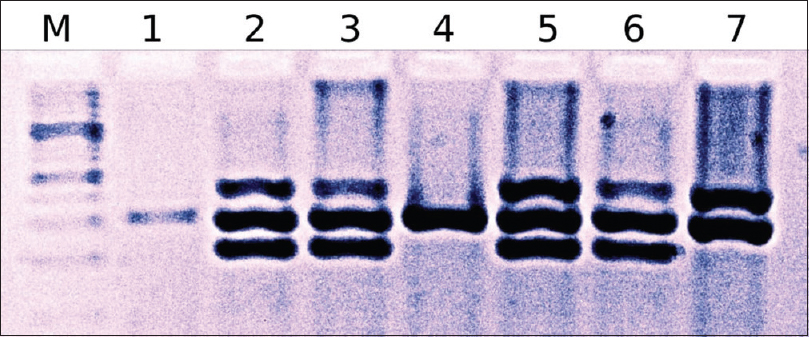 |
| Figure 1: M-100 bp ladder; Lane 1 and 4: Glutathione S-transferase M1 and glutathione S-transferase T1 null; Lane 2, 3, 5, and 6: Glutathione S-transferase M1 and glutathione S-transferase T1 positive, and in Lane 7: Glutathione S-transferase M1 null and glutathione S-transferase T1 positive |
Statistical analysis
Statistical analysis was performed using SPSS software version 20.0 (Chicago). Descriptive measures such as mean and standard deviation were applied for normally distributed variables and t-test for comparison between groups. Binary logistic regression model (BLRM) assessed differences in genotype prevalence and association between cases and controls. Multivariate analysis, Chi-square test, correlation coefficient, odds ratio (OR), and its 95% confidence interval (CI) were used to describe the strength of association. A P value of <0.05 was considered to be statistically significant.
Results
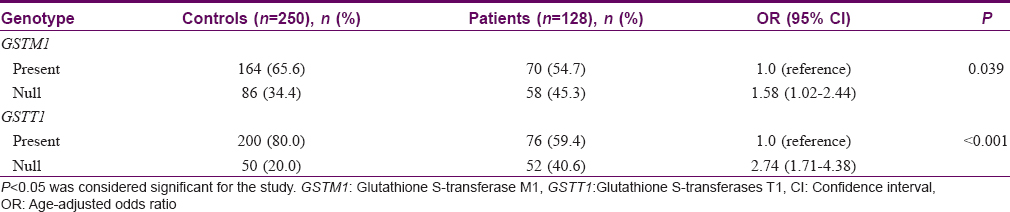
During the course of the study, results of 5% of the samples were checked randomly. Data input and process was double tracked, adopting logic check. [Table - 1] presents the frequencies of GSTM1 and GSTT1 genotypes by case-control status for psoriasis risk. Of the 128 patients with psoriasis, frequency distribution of null genotype of GSTM1 and GSTT1 was 45.3% and 40.6%, respectively while in the 250 control samples, the frequency of null genotype of GSTM1 and GSTT1 was 34.4% and 20.0%, respectively. We observed a significantly higher risk for psoriasis in patients with the null genotype of GSTT1 (OR = 2.74; 95% CI = 1.71–4.38) and GSTM1 (OR = 1.58; 95% CI = 1.02–2.44) as compared to controls [Table - 1]. This association was stronger in patients with the null alleles of GSTT1 (P< 0.001) as compared to GSTM1 null genotypes (P = 0.039).
A combination of the two high-risk genotypes (null genotypes of GSTM1/GSTT1) was also compared to the non-risk genotypes (positive genotypes of GSTM1/GSTT1) for the risk of psoriasis [Table - 2]. The OR for development of psoriasis in the two high-risk genotypes was 3.52-fold higher than the non-risk genotypes (P< 0.001).
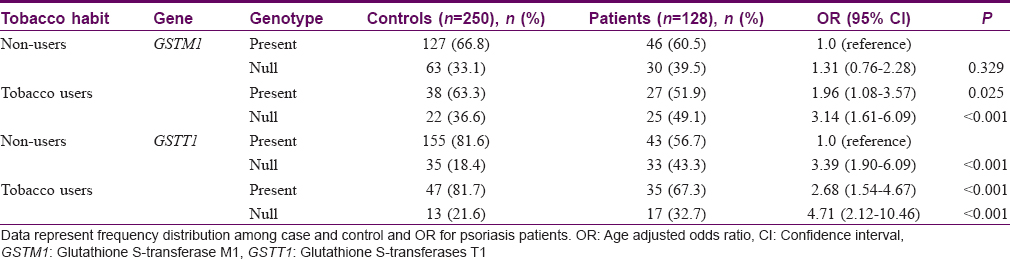
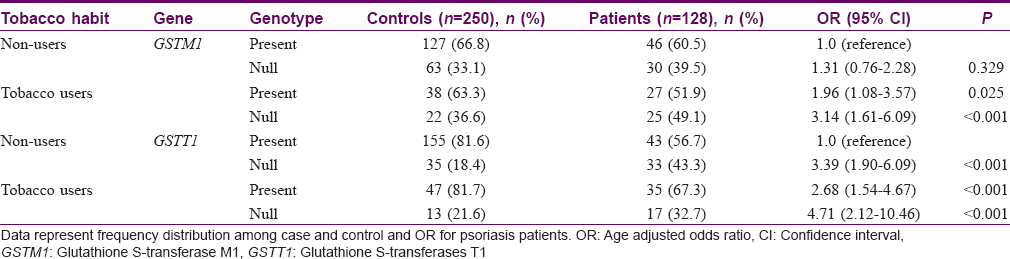
The association between tobacco use and null genotypes of GSTT1 and GSTM1 in patients and controls is summarized in [Table - 3]. Our data indicate that OR for null genotypes of GSTM1 (OR = 3.14) and GSTT1 (OR = 4.71) was higher in tobacco users as compared to positive genotypes of nonusers (P< 0.001).
The association between gender and changes in null genotypes of GSTM1 and GSTT1 in patients and controls is summarized in [Table - 4]. We demonstrated a higher risk in females (3.5-fold) than that in males for GSTT1 null genotypes for susceptibility to psoriasis (OR = 3.53; P < 0.001); however, OR for null genotypes of GSTM1 was statistically nonsignificant (P > 0.05).
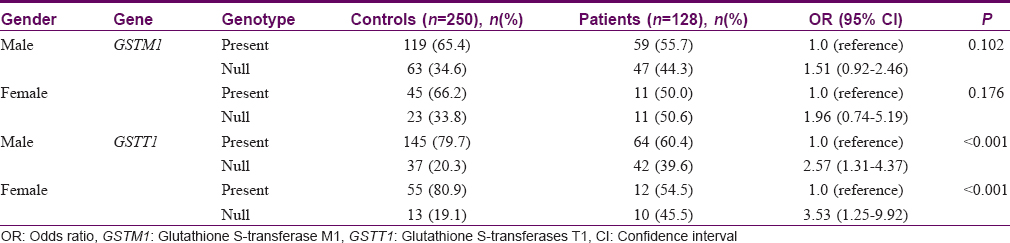
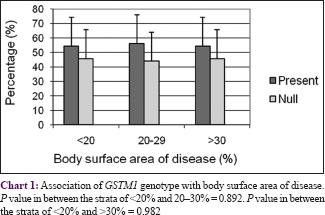
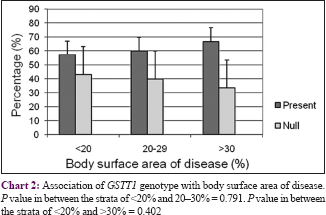
The body surface area affected by psoriasis (grade of disease) was categorized into three groups: <20%, 21–30%, and >30%. There was no correlation between GSTs genotypes and grade or duration of the disease (P > 0.05) with respect to initiation and progression of psoriasis [Chart 1],[Chart 2],[Chart 3],[Chart 4].
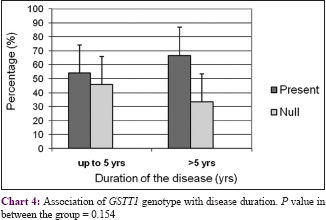
Discussion
In the present study, we found a 34% frequency of null genotypes of GSTM1. This frequency is similar to that reported in African and Southern Asian populations. The 20% frequency observed for null alleles of GSTT1 among healthy individuals also lies within the range reported in Caucasian, European, and South Asian populations [Table - 5].[19],[29],[30],[31]
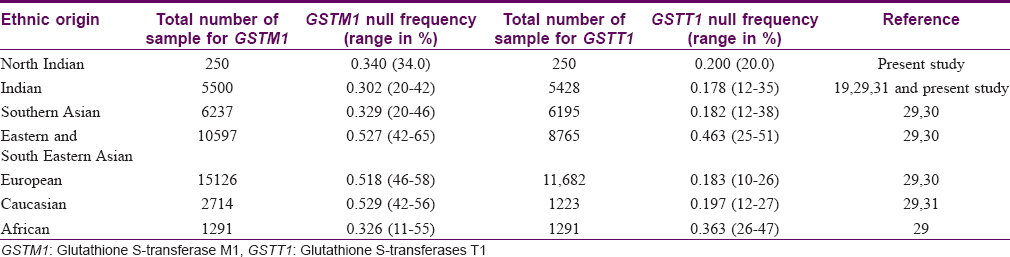
Our study indicated that the null allele of GSTT1 gene is associated with a 2.7-fold higher risk for psoriasis as compared to healthy controls, while the null allele of GSTM1 gene is associated with a lower risk of 1.58 times. No association was observed between GSTs genotypes and the grade or duration of psoriasis. Our findings are in accordance with published reports of the association of GST genes with a number of dermatologic diseases such as psoriasis,[22] solar keratoses,[23] vitiligo,[24] atopic dermatitis,[32] and Behçet's disease.[33] A pioneer study by Richter-Hintz et al. demonstrated that the null alleles of GSTM1 significantly correlated with psoriasis, but not GSTT1.[11] However, a study by Solak et al. in a Turkish population showed no association between GSTM1 and GSTT1 null genotypes with chronic plaque psoriasis.[26] Smith et al. showed that the null genotype of GSTM1 influences erythemal sensitivity to phototherapy in adult Caucasian patients with psoriasis but not the GSTT1 genotypes.[22] Another study showed significant correlation with GSTT1 geno- and phenotypes in psoriasis patients treated with fumaric acid esters but it was not substantially different from healthy controls.[34] Thus, the association of psoriasis risk with null alleles of GSTM1 or GSTT1 varies significantly among different populations.
Patients with both high-risk genotypes of GSTM1 and GSTT1 gene (GSTM1 null and GSTT1 null genotypes) had a 3.5-fold higher risk for psoriasis development compared to positive genotypes of GSTM1 and GSTT1 (P< 0.001, OR = 3.52). Studies have shown that patients with both null alleles of GSTT1/GSTM1 have a higher risk of diseases the urinary bladder,[21] skin,[35] head and neck cancer,[18] type 1 diabetes,[20] and vitiligo[24] when compared to patients with positive alleles of GSTM1/GSTT1 gene.
We also observed a significant association of psoriasis in tobacco users with null genotypes of GSTT1 (OR = 4.71, P < 0.001)/GSTM1 (OR = 3.14, P > 0.001) in comparison to tobacco nonusers with positive genotypes. Tobacco smoke contains 1015–1017 free radicals and other highly reactive electrophiles such as PAH and hydroxylated metabolites of benzo (a) pyrene and GSTs are instrumental in the elimination of these other toxic metabolites thus protecting cells from oxidative stress.[17] Inter-individual variability in the expression of the PAH and BaP metabolizing enzymes may be explained at least in part by genetic polymorphisms in the human genome. Published data suggest that psoriatic individuals with the null allele of GSTM1 who smoke have higher PAH or hydroxylated benzo (a) pyrene genotoxicity and mutagenicity.[4],[5],[8],[9],[11]
Our data also showed a significant association (P< 0.001) of null genotypes of GSTT1 in both males or females with psoriasis risk; however, no association was observed with gender and GSTM1 null genotypes (P > 0.05). This suggests that females who possess null alleles of GSTT1 have higher risk (OR=3.53) for psoriasis as compared to males (OR=2.57) in north Indian population [Table - 4]. This finding is consistent with previous reports in Indian and Chinese populations.[36],[37]
GSTs are phase II xenobiotic metabolizing enzymes active in detoxifying a wide variety of potentially toxic electrophiles by conjugating with glutathione and metabolizing them.[2],[3],[5],[8],[9] A previous study purified and characterized the expression of glutathione transferase in psoriatic skin.[38] GST enzymes play a crucial role in cell protection against oxidative damage which is probably associated with psoriasis.[12],[13],[14],[15],[16],[17] The association with null genotypes of GSTs observed in our study suggests that the inactive form of GSTs enzymes results in reduced detoxification of endogenous/or exogenous toxicants leading to the initiation and progression of psoriasis.
This is the first genetic study in the Indian population exploring the interaction between GSTM1/GSTT1 genotype alone or in combination with tobacco use and susceptibility to psoriasis. To evaluate the interaction between genetic and environmental factors, adequately large sample size is needed. We have examined only two detoxifying genes and further study may be warranted to explore the involvement of other antioxidants and detoxification pathway genes that may be associated alone or in combined analysis in large epidemiological studies.
Conclusion
Our findings indicate that the null allele of GSTM1/GSTT1 genotype alone or in combination with tobacco use are significantly associated with psoriasis risk; however, no association was observed between GSTM1/GSTT1 genotypes and grade/or duration of the disease. Moreover, the presence of both high risk alleles of GST genotypes further augments the risk of psoriasis in the North Indian population.
Acknowledgment
Dr. Daya Shankar Lal Srivastava and Professor V.K Jain conceived, designed, and conducted the study; they also wrote, and edited the manuscript. Dr. J.P. Yadav and Dr. P. Verma helped in performing the experiment. The authors are highly thankful to Vice Chancellor, Pt. B. D. Sharma U.H.S. Rohtak for scientific encouragement and financial support.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370:263-71.
[Google Scholar]
|
| 2. |
Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J Dermatol Sci 2011;63:1-9.
[Google Scholar]
|
| 3. |
Braun-Falco O, Plewig G, Wolff HH, Burgdorf WH. Dermatology. 2nd ed. Berlin: Springer-Verlag; 2000. p. 585-607.
[Google Scholar]
|
| 4. |
Krueger JG, Bowcock A. Psoriasis pathophysiology: Current concepts of pathogenesis. Ann Rheum Dis 2005;64 Suppl 2:ii30-6.
[Google Scholar]
|
| 5. |
Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: Results from an Italian case-control study. J Invest Dermatol 2005;125:61-7.
[Google Scholar]
|
| 6. |
Rashmi R, Rao KS, Basavaraj KH. A comprehensive review of biomarkers in psoriasis. Clin Exp Dermatol 2009;34:658-63.
[Google Scholar]
|
| 7. |
Krishna DR, Klotz U. Extrahepatic metabolism of drugs in humans. Clin Pharmacokinet 1994;26:144-60.
[Google Scholar]
|
| 8. |
Krämer U, Esser C. Cigarette smoking, metabolic gene polymorphism, and psoriasis. J Invest Dermatol 2006;126:693-4.
[Google Scholar]
|
| 9. |
Beranek M, Fiala Z, Kremlacek J, Andrys C, Hamakova K, Chmelarova M, et al. Genetic polymorphisms in biotransformation enzymes for benzo[a] pyrene and related levels of benzo[a] pyrene-7,8-diol-9,10-epoxide-DNA adducts in Goeckerman therapy. Toxicol Lett 2016 25;255:47-51.
[Google Scholar]
|
| 10. |
Pujari VM, Ireddy S, Itagi I, Kumar HS. The serum levels of malondialdehyde, vitamin e and erythrocyte catalase activity in psoriasis patients. J Clin Diagn Res 2014;8:CC14-6.
[Google Scholar]
|
| 11. |
Richter-Hintz D, Their R, Steinwachs S, Kronenberg S, Fritsche E, Sachs B, et al. Allelic variants of drug metabolizing enzymes as risk factors in psoriasis. J Invest Dermatol 2003;120:765-70.
[Google Scholar]
|
| 12. |
Baz K, Cimen MY, Kokturk A, Yazici AC, Eskandari G, Ikizoglu G, et al. Oxidant/antioxidant status in patients with psoriasis. Yonsei Med J 2003;44:987-90.
[Google Scholar]
|
| 13. |
Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol 2003;17:663-9.
[Google Scholar]
|
| 14. |
Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem 2010;25:388-92.
[Google Scholar]
|
| 15. |
Wozniak A, Drewa G, Krzyzynska-Maliniowska E, Czajkowski R, Protas-Drozd F, Mila-Kierzenkowska C, et al. Oxidant-antioxidant balance in patients with psoriasis. Med Sci Monit 2007;13:CR30-3.
[Google Scholar]
|
| 16. |
Abdel-Mawla MY, Nofal E, Khalifa N, Abdel-Shakoor R, Nasr M. Role of oxidative stress in psoriasis: An evaluation study. J Am Sci 2013;9:151-5.
[Google Scholar]
|
| 17. |
Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 1999;49:156-64.
[Google Scholar]
|
| 18. |
Geisler SA, Olshan AF. GSTM1, GSTT1, and the risk of squamous cell carcinoma of the head and neck: A mini-HuGE review. Am J Epidemiol 2001;154:95-105.
[Google Scholar]
|
| 19. |
Mishra DK, Kumar A, Srivastava DS, Mittal RD. Allelic variation of GSTT1, GSTM1 and GSTP1 genes in North Indian population. Asian Pac J Cancer Prev 2004;5:362-5.
[Google Scholar]
|
| 20. |
Vojtková J, Durdík P, Ciljaková M, Michnová Z, Turcan T, Babusíková E. The association between gene polymorphisms of glutathione S-transferase T1/M1 and type 1 diabetes in Slovak children and adolescents. Cent Eur J Public Health 2013;21:88-91.
[Google Scholar]
|
| 21. |
Srivastava DS, Mishra DK, Mandhani A, Mittal B, Kumar A, Mittal RD. Association of genetic polymorphism of glutathione S-transferase M1, T1, P1 and susceptibility to bladder cancer. Eur Urol 2005;48:339-44.
[Google Scholar]
|
| 22. |
Smith G, Weidlich S, Dawe RS, Ibbotson SH. Glutathione S-transferase M1 (GSTM1) genotype but not GSTT1 or MC1R genotype influences erythemal sensitivity to narrow band (TL-01) UVB phototherapy. Pharmacogenet Genomics 2011;21:217-24.
[Google Scholar]
|
| 23. |
Guarneri F, Asmundo A, Sapienza D, Gazzola A, Cannavò SP. Polymorphism of glutathione S-transferases M1 and T1: Susceptibility to solar keratoses in an Italian population. Clin Exp Dermatol 2010;35:771-5.
[Google Scholar]
|
| 24. |
Lu L, Wu W, Tu Y, Yang Z, He L, Guo M. Association of glutathione S-transferase M1/T1 polymorphisms with susceptibility to vitiligo. Gene 2014;535:12-6.
[Google Scholar]
|
| 25. |
Ibbotson SH, Dawe RS, Dinkova-Kostova AT, Weidlich S, Farr PM, Ferguson J, et al. Glutathione S-transferase genotype is associated with sensitivity to psoralen-ultraviolet A photochemotherapy. Br J Dermatol 2012;166:380-8.
[Google Scholar]
|
| 26. |
Solak B, Karkucak M, Turan H, Ocakoglu G, Özemri Sag S, Uslu E, et al. Glutathione S-transferase M1 and T1 gene polymorphisms in patients with chronic plaque-type psoriasis: A case-control study. Med Princ Pract 2016;25:155-8.
[Google Scholar]
|
| 27. |
Sambrook J, Fritsch E, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
[Google Scholar]
|
| 28. |
Abdel-Rahman SZ, Anwar WA, Abdel-Aal WE, Mostafa HM, Au WW. GSTM1 and GSTT1 genes are potential risk modifiers for bladder cancer. Cancer Detect Prev 1998;22:129-38.
[Google Scholar]
|
| 29. |
Kasthurinaidu SP, Ramasamy T, Ayyavoo J, Dave DK, Adroja DA. GST M1-T1 null allele frequency patterns in geographically assorted human populations: A phylogenetic approach. PLoS One 2015;10:e0118660.
[Google Scholar]
|
| 30. |
Kurose K, Sugiyama E, Saito Y. Population differences in major functional polymorphisms of pharmacokinetics/pharmacodynamics-related genes in Eastern Asians and Europeans: Implications in the clinical trials for novel drug development. Drug Metab Pharmacokinet 2012;27:9-54.
[Google Scholar]
|
| 31. |
Sharma A, Pandey A, Sardana S, Sehgal A, Sharma JK. Genetic polymorphisms of GSTM1 and GSTT1 genes in Delhi and comparison with other Indian and global populations. Asian Pac J Cancer Prev 2012;13:5647-52.
[Google Scholar]
|
| 32. |
Cho HR, Uhm YK, Kim HJ, Ban JY, Chung JH, Yim SV, et al. Glutathione S-transferase M1 (GSTM1) polymorphism is associated with atopic dermatitis susceptibility in a Korean population. Int J Immunogenet 2011;38:145-50.
[Google Scholar]
|
| 33. |
Tursen U, Tamer L, Eskandari G, Kaya TI, Ates NA, Ikizoglu G, et al. Glutathione S-transferase polymorphisms in patients with Behçet's disease. Arch Dermatol Res 2004;296:185-7.
[Google Scholar]
|
| 34. |
Gambichler T, Kreuter A, Susok L, Skrygan M, Rotterdam S, Höxtermann S, et al. Glutathione-S-transferase T1 genotyping and phenotyping in psoriasis patients receiving treatment with oral fumaric acid esters. J Eur Acad Dermatol Venereol 2014;28:574-80.
[Google Scholar]
|
| 35. |
Hsu LI, Wu MM, Wang YH, Lee CY, Yang TY, Hsiao BY, et al. Association of environmental arsenic exposure, genetic polymorphisms of susceptible genes, and skin cancers in Taiwan. Biomed Res Int 2015;2015:892579.
[Google Scholar]
|
| 36. |
Srivastava DS, Kumar A, Mittal B, Mittal RD. Polymorphism of GSTM1 and GSTT1 genes in bladder cancer: A study from North India. Arch Toxicol 2004;78:430-4.
[Google Scholar]
|
| 37. |
Ma QW, Lin GF, Chen JG, Shen JH. Polymorphism of glutathione S-transferase T1, M1 and P1 genes in a Shanghai population: Patients with occupational or non-occupational bladder cancer. Biomed Environ Sci 2002;15:253-60.
[Google Scholar]
|
| 38. |
Aceto A, Martini F, Dragani B, Bucciarelli T, Sacchetta P, Di Ilio C. Purification and characterization of glutathione transferase from psoriatic skin. Biochem Med Metab Biol 1992;48:212-8.
[Google Scholar]
|
Fulltext Views
3,569
PDF downloads
2,355





